Oxygen Supplementation Techniques: Best Practices and Considerations
For most organisms on Earth, oxygen is essential for the conversion of food into energy, and is widely utilised in human and veterinary medicine for patient support e.g. oxygen supplementation to delay the onset of hypoxaemia consequential to general anaesthetic-induced hypoventilation or apnoea; or to manage respiratory distress.
In this article we will discuss oxygen supplementation techniques and tips on ensuring what we think we are providing is what we are actually providing.
Introduction
A continuous supply of oxygen is necessary to ensure cellular metabolism and function are maintained, with the brain and heart muscle the most sensitive to hypoxaemia.
In most healthy patients oxygen saturation (SpO2) is maintained at around 97-98%. The aim of oxygen therapy is to maintain SpO2 >90%, equivalent to a partial pressure of oxygen (PaO2) of >60mmHg.
Whilst general anaesthesia or sedation may depress respiration, in anaesthetised, healthy, spontaneously breathing animals, a fraction of inspired oxygen (FiO2) of 0.3 to 0.4 (30 – 40%) or greater is generally adequate to maintain an SpO2 of 97-98% (Martin-Flores et al., 2018). Indeed, very high FiO2 during anaesthesia have been associated with absorption atelectasis, where the high alveolar oxygen concentration rapidly diffuses into the pulmonary circulation resulting in alveolar collapse (Burger & Macklem, 1968), and impairment of oxygenation. However, in a study by Martin-Flores et al., (2020), post-operative hypoxaemia was more commonly associated with FiO2 of 0.4 compared to FiO2 of >0.9 in mechanically ventilated dogs. As most perioperative deaths occur during recovery, especially the first three hours when monitoring may be reduced (Brodbelt et al., 2008) the benefits of decreasing intra-operative FiO2 to reduce atelectasis must be balanced against the risks of post-operative hypoxaemia.
Benefits versus risks
Careful management of patient stress is essential. Regardless of the oxygen delivery system, if that technique causes stress and sympathetic stimulation, the resultant increase in heart and respiratory rate can significantly magnify myocardial oxygen demand. This may be further exacerbated by patient struggling, shivering, and by some premedicants and general anaesthetics.
Delivery techniques
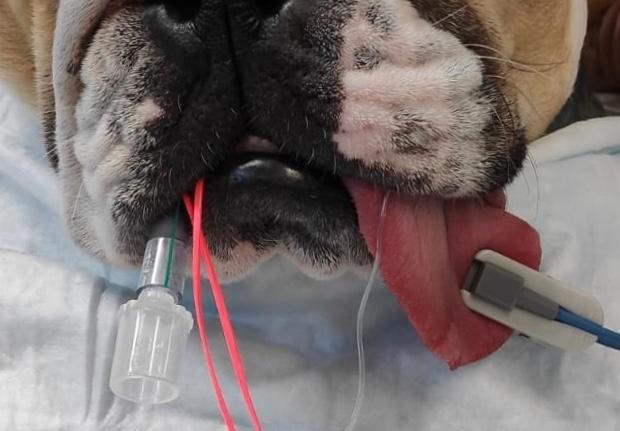
FacemaskFacemasks are one of the most commonly utilised oxygen delivery systems. They are readily available and simple to use (Loukopoulos and Reynolds 1996; Manning 2002; Boyle 2012) and successfully increase FiO2 to above atmospheric levels (Waddell and King, 2018). In healthy, sedated, non-intubated patients, an FiO2 of >0.3 has been reported to minimise the hypoxaemia due to premedicant-induced respiratory depression (Murrell, 2016), and 100% oxygen delivered by facemask at 3L/minute for 5 minutes to healthy sedated dogs can raise PaO2 to 371mmHg (Wong et al., 2018). Indeed, it is possible to achieve an FiO2 of 0.7-0.8 with 100% oxygen at flow rates of 6-8L/minute via facemask (Waddell and King, 2018).
To be effective, the mask should be tight-fitting, but with enough ventilation to allow release of pressure and exhaled carbon dioxide e.g. by removing the rubber diaphragms or use of vented masks (Mosing, 2016). Care must be taken to ensure the masks or diaphragms don’t cause eye trauma, especially in brachycephalics, cats and rabbits. Clear masks are preferred as eye contact can be visualised. Additionally, eye lubrication should be applied to minimise corneal desiccation caused by the cold, dry fresh gas flow.
However, the benefits of oxygen delivery via facemask should be weighed against the risks and only considered if tolerated by the patient. Unfortunately, it is often the patients with the greatest need for oxygen therapy that are the most distressed, and alternative delivery systems may need to be considered in these cases.
Flow-byAlthough flow-by equipment is freely accessible and user-friendly, the technique is considerably less effective than facemask oxygen delivery, with an FiO2 of just 0.3 when 100% oxygen is delivered 2.5cm from the nostrils compared to an FiO2 of 0.9 for facemask delivery (Ambros et al., 2018). Additionally, PaO2 and time to desaturation following apnoea are considerably lower with flow-by than facemask: In the study comparing flow-by and facemask techniques, 3-minutes of 100% oxygen delivered pre-anaesthesia to healthy dogs resulted in a PaO2 of 133 vs 394mmHg; and a time to desaturation of 66 vs187 seconds for flow-by and facemask respectively (Ambros at al, 2018).
Although potentially less stressful than facemask oxygenation, it should also be noted that flow-by becomes significantly less effective if the distance from the delivery system and nostrils increases e.g. should the patient move. Loukopoulos & Reynolds (1997), demonstrated an FiO2 of 0.37 with 100% oxygen delivered 2cm from the nostrils, but when delivered 4cm away the results became unreliable, illustrating the problems associated with patient movement.
Whilst some patients may tolerate flow-by better than facemask oxygenation, if the gas is directed 90o to their nostrils, this may further improve acceptance.
Elizabethan collarUseful for critical patients and for some post-anaesthetic cases, an Elizabethan collar with the lower 2/3 of the opening covered with cling film, and oxygen delivered by a feed tube inserted close to the collar, can produce an effective personal oxygen tent. However, the environment in the collar can become hot and humid, therefore the top 1/3 must be left open to vent carbon dioxide, heat and moisture. As for facemask oxygenation, the eyes should be lubricated and the device only used if tolerated by the patient.
Oxygen cages and tentsFor patients that don’t require regular handling, high oxygen concentrations can be achieved in oxygen cages. However, the oxygen will be lost almost as soon as the door is opened, which negates the effectiveness of the device for patients requiring regular hands-on therapy.
The oxygen cage has limitations: for a regular cat kennel (typical volume of 210 litres), it takes approximately 60 minutes to achieve an FiO2 of 0.5 at flow rates of 2-litres/minute. If the flow rate is increased to 10 litres/minute, the time to reach an FiO2 of 0.5 is only 8 minutes. Due to the limitations of cage size, only patients ≤15kg are suitable.
Although the seals surrounding the cage should ensure a suitable FiO2 is achieved, there must be some method of venting carbon dioxide, heat and humidity. Unfortunately, some oxygen cages are unable to vent appropriately, leaving patients at risk of hypercapnia and hyperthermia, both of which can cause an increase in respiratory effort. Whilst some cages are equipped with carbon dioxide scrubbing via positive air flow systems, simply placing a canister of soda lime in the cage will have no effect.
Oxygen levels in the cage can be readily monitored by oxygen analysers inbuilt in some models, or by using a stand-alone oxygen analyser, such as the ICON. As carbon dioxide is heavier than oxygen, recumbent patients may lie in the region of high CO2 density at the bottom of the cage and not benefit from the oxygen in the higher areas. Therefore, it is essential to position the oxygen analyser at the height of the patient’s head to ensure assessment of the FiO2 at patient level.
Providing oxygen via a cage or tent is not without risk: oxygen is highly flammable, and the high concentration and large volume in the cage present a risk of explosion. Additionally, oxygen supplied from a cylinder can be expensive, although the use of an oxygen concentrator will negate this expense.
Nasal ProngsAlthough nasal prongs produced for human medical purposes may be suitable for longer-term oxygen therapy, they are rarely used for preoxygenation and are not generally well tolerated in health, conscious patients.
Nasal prongs can be placed approximately 1cm inside the nares or, alternatively, feeding tubes (e.g. size 3-10Fg depending on animal size) can be adapted for oxygen delivery and placed uni- or bilaterally in the ventral meatus to the depth of the medial canthus (Bach, 2018). Unilateral nasal cannulae can supply a FiO2 of 0.3 – 0.58 at flows of 50 - 200ml/kg/min, and bilateral cannulae can supply a FiO2 of 0.36 – 0.77 at similar flow rates (Dunphy et al., 2002).
If oxygen is administered via nasal prongs or cannulae, the cold, dry gas should be humidified to minimise the risk of turbinate desiccation and mucosal jet lesions (Bach, 2018). Bubble humidifiers may be utilised to minimise the drying effects on nasal tissues, although the gas won’t generally be warmed (Hughes, 2016).
For mouth-breathing patients, those with nasal discharge, or where sneezing is contraindicated e.g. those with, or where it is necessary to avoid, increased intraocular or intracranial pressure nasal prongs and cannulae should be avoided. Nasal cannulae should also not be used in brachycephalic patients.
Pre-oxygenation
Although some premedicant drugs can cause respiratory depression, apnoea is often related to induction of general anaesthesia, especially if intravenous induction agents are administered rapidly. The aim of pre-oxygenation is to preserve haemoglobin oxygen saturation during these periods of apnoea or hypoventilation until the airway can be secured. In studies of healthy dogs, 100ml/kg/minute of 100% oxygen delivered by facemask was reported to delay the onset of hypoxaemia (SpO2 ≤ 90%) for 3 minutes in apnoeic anaesthetised dogs (McNally et al., 2009), and it is currently accepted practice to preoxygenated for at least 3 minutes prior to induction of anaesthesia. However, should a preoxygenated patient begin breathing room air, the high oxygen concentration in the alveoli is rapidly displaced within a few breaths, negating the benefits of the supplemental oxygen. It is for this reason that oxygen cages are not suitable for preoxygenation.
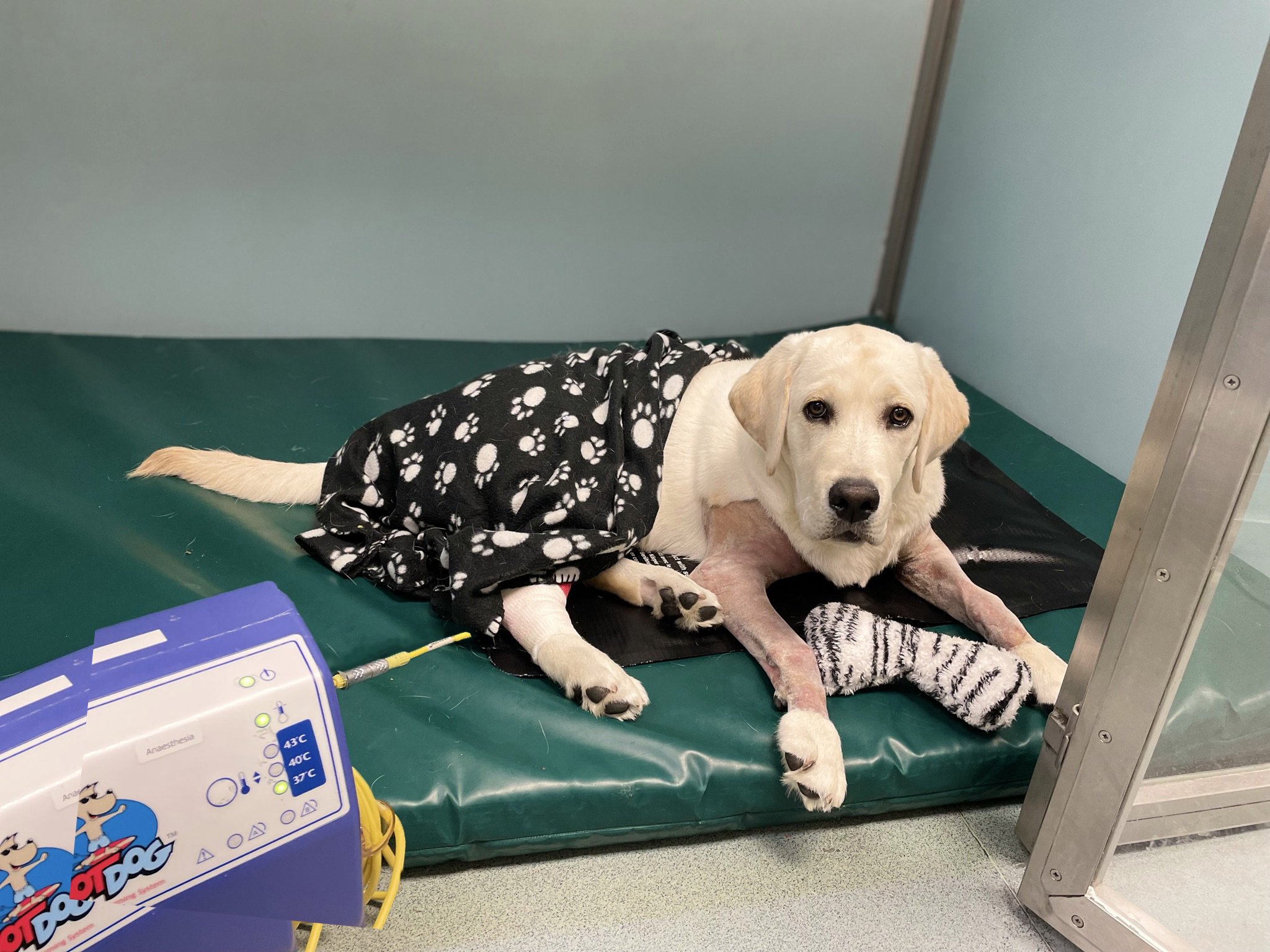
Post-operative oxygen therapy
The first three hours of recovery are the most critical with regards to the incidence of perioperative death (Brodbelt et al., 2008). During this period, respiratory depression as a consequence of residual anaesthetic drugs; increased oxygen demand following hypothermia-induced shivering; atelectasis; or partial airway obstruction from relaxed, excessive or inflamed tissues may all decrease SpO2. As cyanosis is not apparent until SpO2 of 80-85% (PaO2 of 50mmHg), it is essential to continue monitoring during this critical post-operative period. Pulse oximetry is a readily available, simple technique that is especially valuable for alerting clinical personnel when patients are experiencing SpO2 ≤90% (PaO2 of ≤60mmHg) i.e. before cyanosis is observed. To guide expectations during recovery, a pre-anaesthetic SpO2 should ideally be obtained, especially in brachycephalics that may have a “normal” SpO2 of 90-95%.
Oxygen Toxicity
Whilst uncommon in veterinary patients, oxygen therapy at FiO2 >0.6 (60%) for more than 12 hours can cause symptoms of oxygen toxicity e.g. coughing and respiratory distress, caused by excessive production of reactive oxygen species (ROS), such as free radicals. These ROS can damage proteins, lipids and DNA, resulting in inflammation and lung damage (Waddell and King, 2018). Consequently, for mid- to long-term therapy the FiO2 should be reduced to the lowest effective concentration.
Conclusions
There are multiple techniques for providing additional oxygen to our veterinary patients. Selection of the most appropriate method is dependent on an understanding of the reasons for supplying supplementary oxygen, the delivery system itself, and the specific requirements and cooperation of the patient.
References
Ambros B., Carrozzo M.V. & Jones T. (2018). Desaturation times between dogs preoxygenated via face mask or flow-by technique before induction of anesthesia. VAA, 45: 452–458 https://doi.org/10.1016/j.vaa.2018.03.004
Bach, J. (2018). Chapter 15, Oxygen Delivery Systems. In Cooley K. G. & Johnson R. A. (eds), Veterinary Anesthetic and Monitoring Equipment. Wiley Blackwell, Hoboken, New Jersey, USA. pp 193 - 198
Boyle J. (2012). Oxygen therapy. In: Burkitt Creedon JM, Davis H (eds). Advanced Monitoring and Procedures for Small Animal Emergency and Critical Care. pp 263–73. Wiley Blackwell, Ames, IA, USA
Brodbelt D.C., Blissett K.J., Hammond R.A. Neath P.J., Young L.E., Pfeiffer D.U. & Wood J.L.N. (2008). The risk of death: the Confidential Enquiry into Perioperative Small Animal Fatalities. VAA, 35: 365-373 httpsdoi: https://doi.org/10.1111/j.1467-2995.2008.00397.x
Burger E.J. Jr., Macklem P. (1968). Airway closure: demonstration by breathing 100 percent O2 at low lung volumes and by N2 washout. J Appl Physiol, 25: 139–148.
Dunphy E.D., Mann F.A., Dodam J.R., Branson K.R., Wagner-Mann C.C., Johnson P.A. & Brady M.A. (2002). Comparison of unilateral versus bilateral nasal catheters for oxygen administration in dogs. JVECC, 12: 245–251
Hughes L. (2016). Chapter 5. Breathing systems and ancillary equipment. In: Duke-Novakovsky T., de Vries M. & Seymour C. (eds). BSAVA Manual of Canine and Feline Anaesthesia and Analgesia. British Small Animal Veterinary Association, Gloucester, UK. pp 45-64
Loukopoulos P, Reynolds W. (1996). Comparative evaluation of oxygen therapy techniques in anaesthetised dogs: intranasal catheter and Elizabethan collar canopy. Aus Vet Prac, 26: 199–205
Loukopoulos P. & Reynolds W. (1997). Comparative evaluation of oxygen therapy techniques in anaesthetised dogs: face mask and flow-by technique. Aus Vet Prac, 27: 34–9
Manning AM. (2002). Oxygen therapy and toxicity. VCNA:SAP 32: 1005–1020
Martin-Flores M., Tseng C.T., Robillard S. D., Abrams B.E., Campoy L., Harvey H. J. & Gleed R. D. (2018). Effects of two fractions of inspired oxygen during anesthesia on early postanesthesia oxygenation in healthy dogs. AJVR, 79(2): 147-153. https://doi.org/10.2460/ajvr.79.2.147
Martin-Flores M., Cannarozzo C.J., Tseng C.T., Lorenzutti A.M., Araos J.D., Harvey H.J., Gleed R.D. & Campoy L. (2020). Postoperative oxygenation in healthy dogs following mechanical ventilation with fractions of inspired oxygen of 0.4 or >0.9. VAA, 47: 295-300. https://doi.org/10.1016/j.vaa.2020.01.002
McNally, E. M., Robertson, S. A. and Pablo, L. S., (2009). Comparison of time to desaturation between preoxygenated and non preoxygenated dogs following sedation with acepromazine maleate and morphine and induction of anesthesia with propofol. AJVR, 70(11): 1333–1338 https://doi.org/10.2460/ajvr.70.11.1333
Mosing M. (2016). General principles of perioperative care. In: Duke-Novakovsky T., de Vries M. & Seymour C. (eds). BSAVA Manual of Canine and Feline Anaesthesia and Analgesia. British Small Animal Veterinary Association, Gloucester, UK. pp 13–23 https://doi.org/10.22233/9781910443231.3
Murrell, J. C., (2016). Pre-anaesthetic medication and sedation. In: Duke-Novakovsky T., de Vries M. & Seymour C. (eds). BSAVA Manual of Canine and Feline Anaesthesia and Analgesia. British Small Animal Veterinary Association, Gloucester, UK. pp. 170–189 https://doi.org/10.22233/9781910443231.13
Waddell L.S. and King L.G. (2018). General approach to respiratory distress. In: BSAVA Manual of Canine and Feline Emergency and Critical Care. British Small Animal Veterinary Association, Gloucester, UK. pp. 93–122 https://doi.org/10.22233/9781910443262.7
Wong, A., Uquillas, E., Hall, E., Dart, C. and Dart, A., (2018). Comparison of the effect of oxygen supplementation using flow-by or a face mask on the partial pressure of arterial oxygen in sedated dogs. NZ Vet J, 67(1): 36–39. https://doi.org/1080/00480169.2018.1528903

 By
By