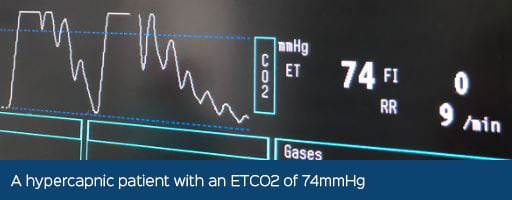
Are you providing effective Oxygen Therapy?
Many of our veterinary patients may require oxygen support and supplementation for various reasons, e.g. respiratory distress, heart failure, or pre-oxygenation before anaesthesia to treat or delay the onset of hypoxaemia. Oxygen can be provided via flow-by, mask, nasal prongs or with the patient sitting in an oxygen cage. Transtracheal administration can also be performed, but it is less commonly performed in general practice and it is not discussed further.
Unfortunately, unless oxygen supplementation is performed correctly, all efforts may be in vain if the equipment is not used correctly and whilst we think we are providing effective treatment, our patients may not be receiving a worthwhile concentration of oxygen. Additionally, some techniques may not be tolerated by the patient and can actually cause them to have an increased oxygen demand e.g. fighting restraint.
It is recommended that in a patient with a respiratory compromise that is receiving supplemental oxygen and where their Partial Pressure of Arterial Oxygen (PaO2) remains <50-60mmHg with a Fraction of Inspired Concentration of Oxygen (FiO2) of >0.6, then the patient may have to be anaesthetised and ventilated (Grubb, 2016).
In this article, we discuss different techniques in oxygen supplementation and ways to make sure what you’re providing to your patients is what you suspect.
Pre-oxygenation
Providing oxygen to a patient prior to the induction of anaesthesia is known as pre-oxygenation, where the aim is to create an oxygen-rich environment in the alveoli for gas exchange to continually occur in case there is post-induction apnoea or a delay in securing the airway e.g. excess pharyngeal tissue in brachycephalic patients causing an airway obstruction resulting in hypoxaemia. Providing 100% oxygen delivered by a facemask at 100ml/kg/minute for 3 minutes can delay the onset of hypoxaemia for up to 5 minutes (McNally et al., 2009). Preoxygenation should be avoided in cases where it causes stress to the patient as its benefits are outweighed.

A French Bulldog being pre-oxygenated prior to anaesthesia, using a laryngoscope to depress the tongue and free the epiglottis.
However, where pre-oxygenation is performed, it is important to note that even when 100% of oxygen is being used, the patient will not receive this concentration. For example, room air has a FiO2 of 0.21 or 21%, but delivering 100% oxygen via a breathing system placed within 2 cm of the patient’s nose (flow-by) can only increase the FiO2 to up 0.4 (40%) (Mosing, 2016), and if using a tight-fitting face mask, it can increase the FiO2 up to 0.7-0.8 (Waddell and King 2018).
The correct breathing system must be used to perform pre-oxygenation. It can be provided with all types of non-rebreathing systems, however, not all circle breathing systems will have oxygen flow down the inspiratory limb as it depends on where the fresh gas flow enters the system – either behind the inspiratory valve or on the patient side of the valve. To eliminate any confusion as to what type of circle system you have, the APL valve can be closed whilst delivering flow-by and reopened at the time of anaesthesia induction.
Preoxygenation via an oxygen cage is not effective as once the patient is removed, the highly concentrated alveoli gas is displaced by room air within a few breaths.
Flow-by Oxygen
As previously mentioned, flow-by is not very effective at raising the concentration of oxygen being provided to a patient as the FiO2 rarely exceeds 0.4, even when placed as close as 2 cm from the nose (Mosing, 2016). It also is immediately ineffective once the patient turns their head.
Additionally, if a patient is being sedated and is not orotracheally intubated, increasing the FiO2 to >0.3 will prevent hypoxaemia that may occur with respiratory depression (Murrell 2016). This can be performed with flow-by in close proximity to the nose or with a facemask.
If flow-by oxygen support is being provided, some patients tolerate the breathing system being aimed at their nose at a 90° angle versus head-on due to the feeling of being blown in the face.
Lastly, flow-by oxygen support may not be effective if the patient is panting heavily.
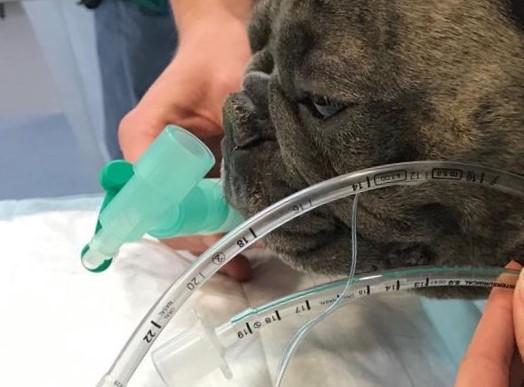 A French Bulldog being pre-oxygenated whilst measuring and comparing lengths of endotracheal tubes.
A French Bulldog being pre-oxygenated whilst measuring and comparing lengths of endotracheal tubes.
Masked Oxygen
Effective oxygen therapy can be provided via a facemask if the patient tolerates it. Care should be taken to accidental trauma to the patient’s eyes in brachycephalic breeds. The patient should also not become stressed by this technique as the benefits become outweighed by the stress, and may not be tolerated in some patients that require it the most during emergency stabilisation e.g. respiratory distressed brachycephalic patients.
If the mask is tight fitting with oxygen flow rates of 6-8L/minute, an FiO2 of 0.7-0.8 may be achieved (Waddell and King 2018). One study found that providing 100% oxygen via a facemask for 5 minutes at 3L/minute raised the PaO2 to 371mmHg (Wong et al., 2018). Additionally, the mask shouldn’t be tight so that there is rebreathing of carbon dioxide and therefore it is often recommended that the black diaphragm is removed if it is too tight-fitting.
Similar to masked oxygen, an Elizabethan collar can be placed around the patient and sealed with clingfilm with a small oxygen tube feeding into the enclosed space. A small space, a third of the collar, must be left at the top of the clingfilm for carbon dioxide to be vented. This technique may not be tolerated well by all patients, and the environment in the collar may become hot and humid, making the patient pant more, limiting its success. Eye lubrication should also be applied to prevent them from drying out, especially if opioids have also been administered.
Oxygen Cages
The use of oxygen cages in critical care medicine is favourable as handling of the patient is minimalised and high concentrations of oxygen can potentially be reached. Limitations however include that when the cage is opened, the high-concentration environment is lost within seconds, some critical patients may need more hands-on treatment and additionally, this method of oxygen support is usually only suitable for patients up to 15kg due to cage size limitations.
It cannot be stressed enough that the flow rate of oxygen into the oxygen cage is sufficient and the frequency of the opening of the cage is kept to a minimum. It is worthwhile to consider the measurements of the cage against the flow rate being provided e.g. a cage that measures 71 cm x 54 cm x 55 cm has a volume of 210L, and at a flow rate of 2L/minute, after 60 minutes, the concentration of oxygen is approximately 52%, compared to a flow rate of 10L/minute, which reaches the same concentration within 8 minutes. To monitor the concentration of oxygen within an oxygen cage, a simple monitor, such as the ICON oxygen analyser can be attached to the cage wall, at the height of the patient’s head. By placing the ICON at the level of the patient, it ensures the oxygen concentration that the patient is inhaling is known as different atmospheric gases have different densities and “sit” at different heights in the cage due to their molecular weights. For example, oxygen has a molecular mass of 32, nitrogen is 28 and carbon dioxide is 44 – meaning that the heavy expiratory breath tends to remain low in the cage, often where a recumbent or obtunded patient is laying.
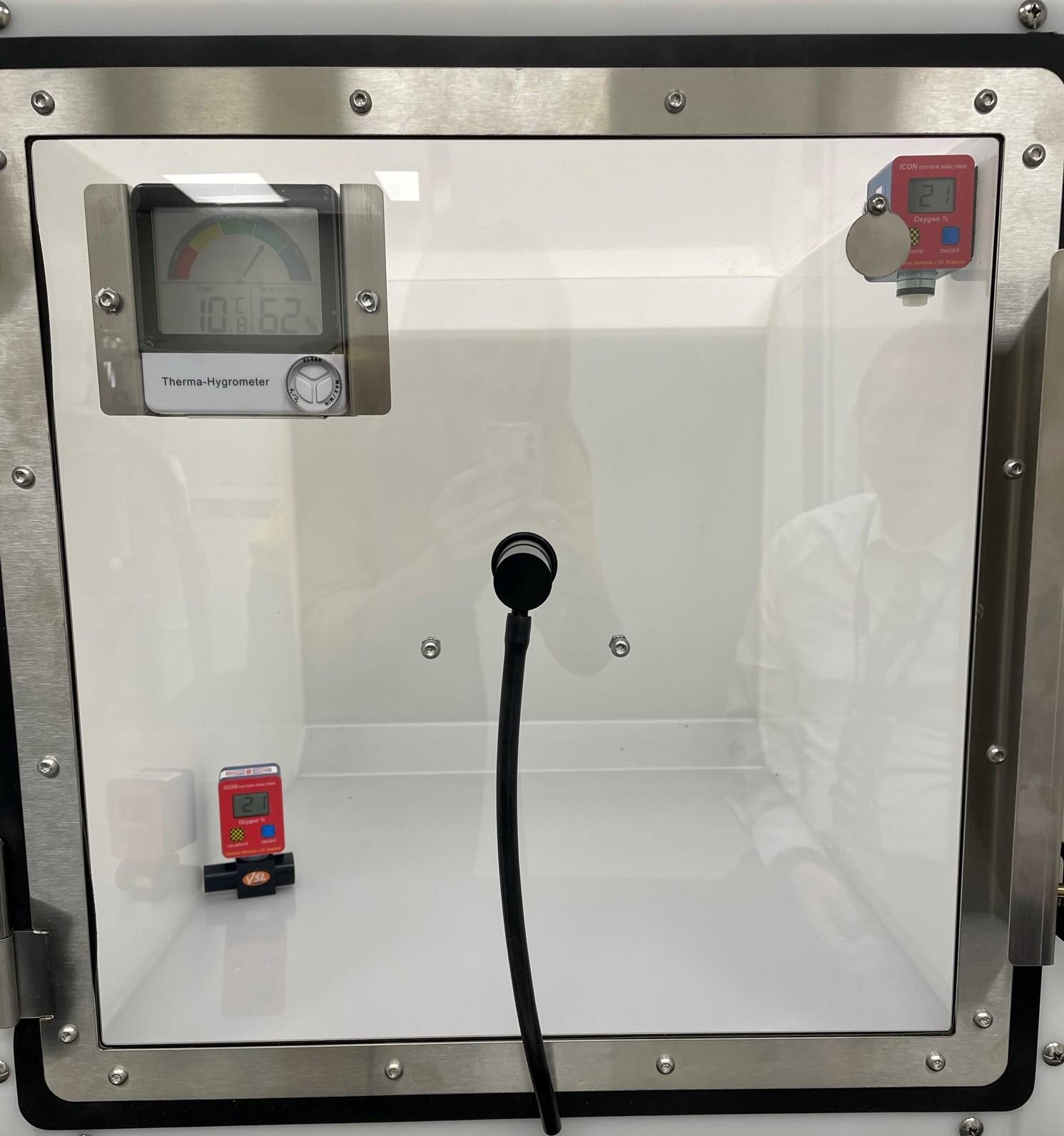
An oxygen cage with two ICON oxygen analysers to see where the highest concentrations are when filled from the middle of the cage.
The seal required around an oxygen cage to ensure a high concentration of oxygen is reached, and the combination of some oxygen cages being unable to effectively monitor and ventilate excessive heat and carbon dioxide, can result in hyperthermia and hypercarbia which increases the patient’s respiratory rate and effort to compensate. Some sophisticated oxygen cage models encourage airflow through a soda lime canister to remove carbon dioxide, however, simply putting a tub of soda lime in an oxygen cage will not be of any benefit.
From an economical perspective, the cost of running an oxygen cage with cylinder oxygen can be expensive and it does not come without the risk of explosion due to the high oxygen concentration being extremely flammable. Many purpose-built oxygen cages will come with a manual explaining how to minimise these risks.
Nasal Prongs
Nasal prongs that are sourced from human medicine can be used in patients that tolerate them, where they sit over their face and sit 1 cm in the nares. Additionally, red rubber urinary catheters (5-8 Fg) can be placed in one or both nostrils to the depth of the medial canthus and sutured in place.
Nasal prongs are not used for pre-oxygenation as they are typically not tolerated in routine cases where they are not already in place and they are not suitable for brachycephalic patients. They also should not be used in patients where sneezing is contraindicated e.g. those patients with an increase in intraocular pressure or intracranial pressure. They are not useful in patients that are mouth-breathing.
With nasal catheters, a FiO2 of 0.4 can be reached depending on the flow rate if placed unilaterally and if placed bilaterally, can provide a FiO2 of approximately 0.6 (Waddell and King, 2018; Dunphy et al., 2002). Zimmerman et al., (2013) found that the FiO2 reached with a unilateral nasal catheter varies with oxygen flow e.g., oxygen flow rates of 50ml/kg/minute produced a FiO2 of 0.39 and at 100 ml/kg/minute, a FiO2 of 0.49 was reached, depending on the patients RR and TV.
Humidification
If oxygen is provided for long periods (a few hours) it should be humidified to protect the respiratory mucosa. The oxygen delivered via a nasal catheter or an oxygen cage can be humidified via a bubble through a humidifier using distilled water.
Oxygen Therapy in the Post-Operative Period
Patients may become hypoxaemic in the recovery period for several reasons, including respiratory depression from residual anaesthetic drugs causing hypoventilation, shivering from hypothermia which causes many muscles to contract that increases oxygen consumption, ongoing atelectasis from patient positioning or partial obstruction from relaxed pharyngeal tissue or airway swelling after intubation.
A pulse oximeter can be placed on the patient in the recovery period or a blood gas sample can be obtained to see if hypoxaemia is present (a SpO2 <90% or a PaO2 <60mmHg). It should be known that cyanosis is not visible until the oxygen saturation is below 85%. If tolerated, a pre-anaesthesia oxygen saturation can be obtained to help guide expectations in the recovery period e.g. a brachycephalic patient may have a “normal” reading of 90-95% before anaesthesia.
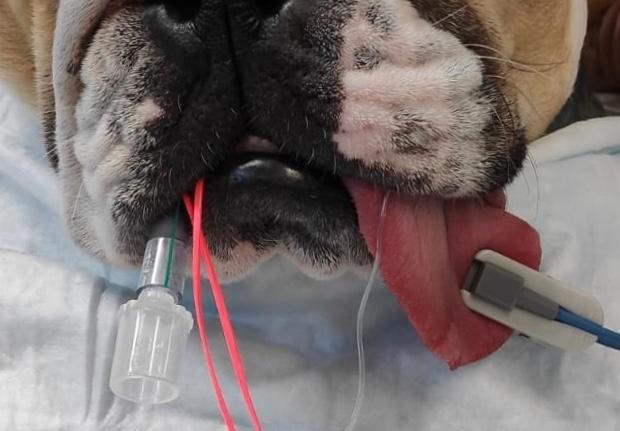
Monitoring a patient’s oxygen saturation during the recovery period.
If you suspect or confirm a patient in recovery may be hypoxaemic, the veterinary surgeon should be notified as oxygen support may be required. It is important to note that diagnosis of hypoxaemia must be made by the VS and treatment prescribed accordingly. Some anaesthetic drugs may need to be antagonised.
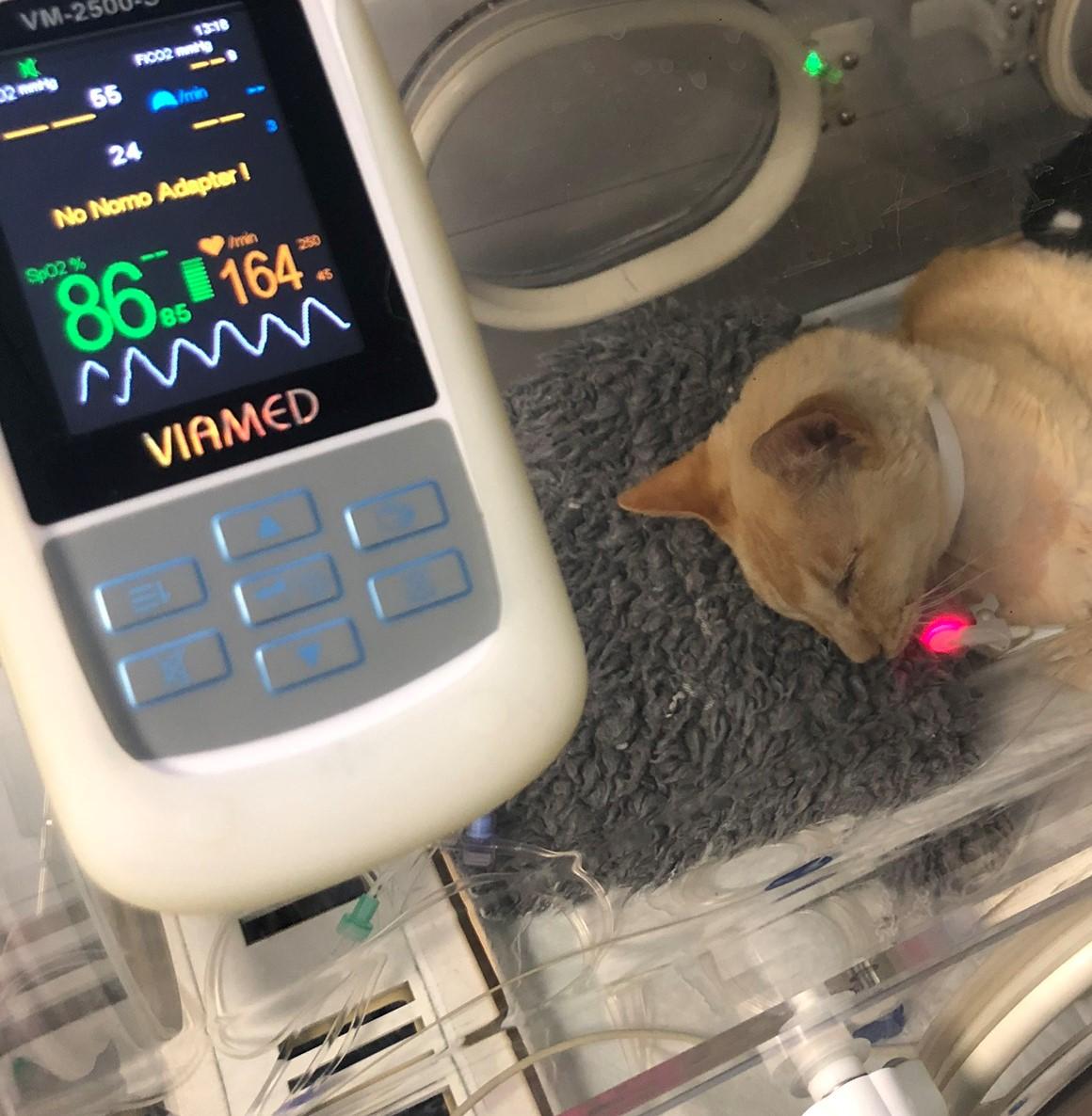
A cat recovering in a baby incubator that is piped with oxygen with a low oxygen saturation, which was known prior to anaesthesia due to their disease process.
Oxygen Toxicity
Whilst uncommon in veterinary anaesthesia, long-term therapy with high concentrations of oxygen (FiO2 >0.6) may result in oxygen toxicity if used for periods for longer than 12 hours. Injury includes damage to the lung itself and inflammatory injury due to toxic metabolites of oxygen such as free radicals and superoxide molecules (Waddell and King, 2018).
Therefore, the FiO2 should be minimised in patients to the lowest effective concentration.
Conclusions
The equipment and methods used to provide oxygen support to patients must be fully understood to make sure it is of benefit to the patient.
Recommended Reading
“Respiratory Compromise” – Chapter 13, BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd Edition.
Test Your Knowledge
- What level of PaO2 defines hypoxaemia?
- <60mmHg
- 60-100mmHg
- 100-150mmHg
- >150mmHg
- When providing flow-by oxygen supplementation, what is the maximum FiO2 reached?
- Up to 0.2
- Up to 0.4
- Up to 0.6
- Up to 0.8
- High concentrations of oxygen are a fire and explosive risk:
- True
- False
References
Grubb, T., (2016). Respiratory compromise. In: BSAVA Manual of Canine and Feline Anaesthesia and Analgesia. British Small Animal Veterinary Association. pp. 314–328. Available from: doi: 10.22233/9781910443231.22
McNally, E. M., Robertson, S. A. and Pablo, L. S., (2009). Comparison of time to desaturation between preoxygenated and non preoxygenated dogs following sedation with acepromazine maleate and morphine and induction of anesthesia with propofol. American Journal of Veterinary Research. 70(11), 1333–1338. Available from: doi: 10.2460/ajvr.70.11.1333
Mosing, M., (2016). General principles of perioperative care. In: BSAVA Manual of Canine and Feline Anaesthesia and Analgesia. British Small Animal Veterinary Association. pp. 13–23. Available from: doi: 10.22233/9781910443231.3
Murrell, J. C., (2016). Pre-anaesthetic medication and sedation. In: BSAVA Manual of Canine and Feline Anaesthesia and Analgesia. British Small Animal Veterinary Association. pp. 170–189. Available from: doi: 10.22233/9781910443231.13
Waddell, L. S. and King†, L. G., (2018). General approach to respiratory distress. In: BSAVA Manual of Canine and Feline Emergency and Critical Care. British Small Animal Veterinary Association. pp. 93–122. Available from: doi: 10.22233/9781910443262.7
Wong, A., Uquillas, E., Hall, E., Dart, C. and Dart, A., (2018). Comparison of the effect of oxygen supplementation using flow-by or a face mask on the partial pressure of arterial oxygen in sedated dogs. New Zealand Veterinary Journal. 67(1), 36–39. Available from: doi: 10.1080/00480169.2018.1528903
Zimmerman, M. E., Hodgson, D. S. and Bello, N. M., (2013). Effects of oxygen insufflation rate, respiratory rate, and tidal volume on fraction of inspired oxygen in cadaveric canine heads attached to a lung model. American Journal of Veterinary Research. 74(9), 1247–1251. Available from: doi: 10.2460/ajvr.74.9.1247