Managing Anaesthesia Related Hypothermia in Cats & Dogs
Hypothermia is a common complication in patients during the anaesthesia period. In this blog, we examine the causes and consequences of hypothermia, its prevention and treatment, and then look at the range of Burtons Medical Equipment products available to help you, help your patients.
Thermoregulation:
Thermoregulation is where the animal can keep its temperature within a certain range, even when the surrounding temperature is different. Heat is a by-product of all metabolic processes, and the maintenance of this core body temperature optimises enzyme function and homeostasis. In conscious animals, the core body temperature (trunk and head) is stable, with variable temperatures in the periphery. The difference between the core and peripheral temperatures allows a gradient for skin to either conserve or dissipate body heat via the arteriovenous anastomoses in the skin (Posner, 2007). As tissue is a poor conductor of heat, it is most efficiently transferred in the blood.
Normal rectal temperature values vary depending on the author, but they are typically within ranges of 38-39°C. On average, the core temperature is 0.4°C higher than rectal temperatures in dogs (Osinchuk et al., 2014). The core temperature can also be up to 4°C less than the peripheral body temperature (Clark-Price, 2015).
Thermoregulation is complex; there is continuous sensing and processing of information from the thermal receptors, transmitted using the same A-fibre and C-fibre pain-sensing neurons, which is received by the hypothalamus where it compares this information to the interthreshold range. The interthreshold range is a narrow range where no thermoregulatory responses are triggered, which is approximately ±0.2°C from the setpoint body temperature. If the core body temperature is above or below the interthreshold range, then behavioural or physiological responses occur to normalise this. Behavioural responses to increasing body temperature include heat-seeking, such as finding a sunny patch to lay in, and physiological responses include piloerection, shivering and vasoconstriction or vasodilation.
Anaesthesia related Hypothermia:
A large-scale study found that only 3% of general practices in the United Kingdom monitored patient temperature during the peri-anaesthesia period, despite it being a very simple patient parameter to obtain (Brodbelt et al., 2008), and that the incidence of post-anaesthetic hypothermia was 97% in cats and 84% in dogs (Redondo et al., 2012ab).
Hypothermia in cats and dogs is defined as slight (38.4-36.5°C), moderate (36.49-34.0°C) or severe (<34.0°C) (Redondo et al., 2012ab). While diagnosing hypothermia within the upper limit of the ‘slight’ category may seem unreasonable, it may instead be appreciated as a temperature decrease of 1°C from the patient’s baseline temperature, prior to anaesthesia.
Anaesthesia has a holistic effect on thermoregulation as there is a loss of behavioural responses to a lower body temperature and the interthreshold range also widens, altering the trigger for the physiological responses to the decline in body temperature.
Cats, small dogs and small mammals (e.g., rabbits), as well as neonatal and geriatric patients, will be more susceptible to hypothermia due to their large surface area to body weight ratio, or their reduced muscle mass and body fat. Animals with hepatic dysfunction are also prone to hypothermia as the heat that is typically generated with its metabolic activity is also reduced.
Side effects of Hypothermia:
Hypothermia can cause a range of negative physiological effects, some of which are minor and some that can become life-threatening.
Complications from hypothermia include bradycardia, hypotension through reduced vascular tone and cardiac output, subjective overdoses of volatile agents due to a reduction in Minimal Alveolar Concentration (MAC), prolonged action of injectable drugs due to reduced metabolism, acid-base disturbances, prolonged clotting times causing blood loss, poor wound healing and in severe cases, cardiac arrhythmias (<30°C).
These complications can all contribute to patient increases in anaesthesia related morbidity and mortality.
Heat Loss:
There are 4 types of heat loss: radiation, convection, conduction and evaporation. Radiation and convection are the biggest concern for our patients during anaesthesia as 80% of heat is lost in this way:
• Radiation is where heat travels in waves away from the body to the cooler environment.
• Convection is when warm air that surrounds the body is carried away by the airflow in the environment or when the air surrounding the body is cooler, such as a cold operating room.
• Conduction heat loss is where heat is transferred to a surface or substance colder than itself, like a patient laying on a cold surgical table.
• Evaporation heat loss can occur from the respiratory tract, but also in patients with large surgical incisions such as exploratory laparotomies. Respiratory heat is worsened with the use of cold dry anaesthetic gases.
Radiation and convection are the biggest concern for our patients during anaesthesia as 80% of heat is lost in this way. Ultimately, all heat losses are dependent on the amount of exposed skin and may be further exacerbated by the clipping of fur in surgical site preparation, as fur is a natural insulator.
There are three phases to heat loss, which typically follow a defined pattern:
1. Phase one occurs within the first hour of anaesthesia. Body heat from the core is distributed to the periphery, resulting in a large drop in patient temperature. This is a critical period to provide patient warming - from premedication through to induction of anaesthesia and surgical site preparation; the warmer the peripheral temperature, the less redistribution of heat from the core will occur.
2. The second phase is where there is a slower linear drop in core temperature for the next 2-3 hours as there is an increase in heat loss in excess of heat production.
3. Then there is a thermal plateau, where the core temperature remains fairly unchanged as heat loss will equal heat production.
What contributes to Hypothermia?
The patient’s temperature decreases due to reduced muscle activity, a reduced metabolic rate, anaesthetic drug-induced dose-dependent depression of the thermoregulatory centre and peripheral vasodilation.
There are some anaesthesia drugs that may contribute directly to hypothermia. Acepromazine has an effect on ⍺-adrenergic receptors, leading to a degree of vasodilation which may cause hypothermia due to heat loss from the periphery. One consideration with local regional blocks, especially across large sites (e.g., an epidural), is that vasodilation will occur, and the patient will lose the ability to shiver in that area. Volatile agents also cause hypothermia through vasodilation, as well as depression of the thermoregulatory centre.
In addition to this, hypothermia may arise due to excessive surgical site clipping, skin preparation liquid, ambient temperature intravenous (IV) fluid therapy administration, cold operating tables, cool ambient air temperature and cold gases being administered through non-rebreathing systems, as previously mentioned.
Redondo et al. (2012) identified other variables associated with a decrease in the patient’s post-operative temperature, including:
• Duration of the pre-anaesthetic time
• Duration of the anaesthetic
• Physical condition – Patients with an ASA III-IV had lower temperatures than ASA I dogs
• Procedure type – Diagnostic and thoracic procedures reduced the temperature compared to minor procedures in dogs, and abdominal and orthopaedic surgeries significantly reduced the temperature compared to minor procedures in cats
• Recumbency – Dogs in sternal and dorsal recumbency have lower temperatures than lateral recumbency

Monitoring Hypothermia:
Many multiparameter machines will display a continuous reading of a patient’s body temperature when probes are inserted into the oesophagus or rectum. Inadvertent placement of the oesophageal thermometer probe into the stomach can reduce the accuracy of temperature readings. Alternatively, handheld digital thermometers can obtain rectal, nasal or tympanic membrane temperatures.
There is mixed evidence on the reliability of readings obtained by the tympanic membrane and rectal temperatures may lag behind changes in core body temperature.
Unless the patient's temperature is monitored continuously with a multiparameter, it is advised to obtain a reading every 15 minutes and act on the trends. If hypothermia is noted or the patient’s temperature is trending down, active warming should commence. Termination of active warming is recommended when the patient is 1°C from the desired temperature, avoiding advertent overheating.
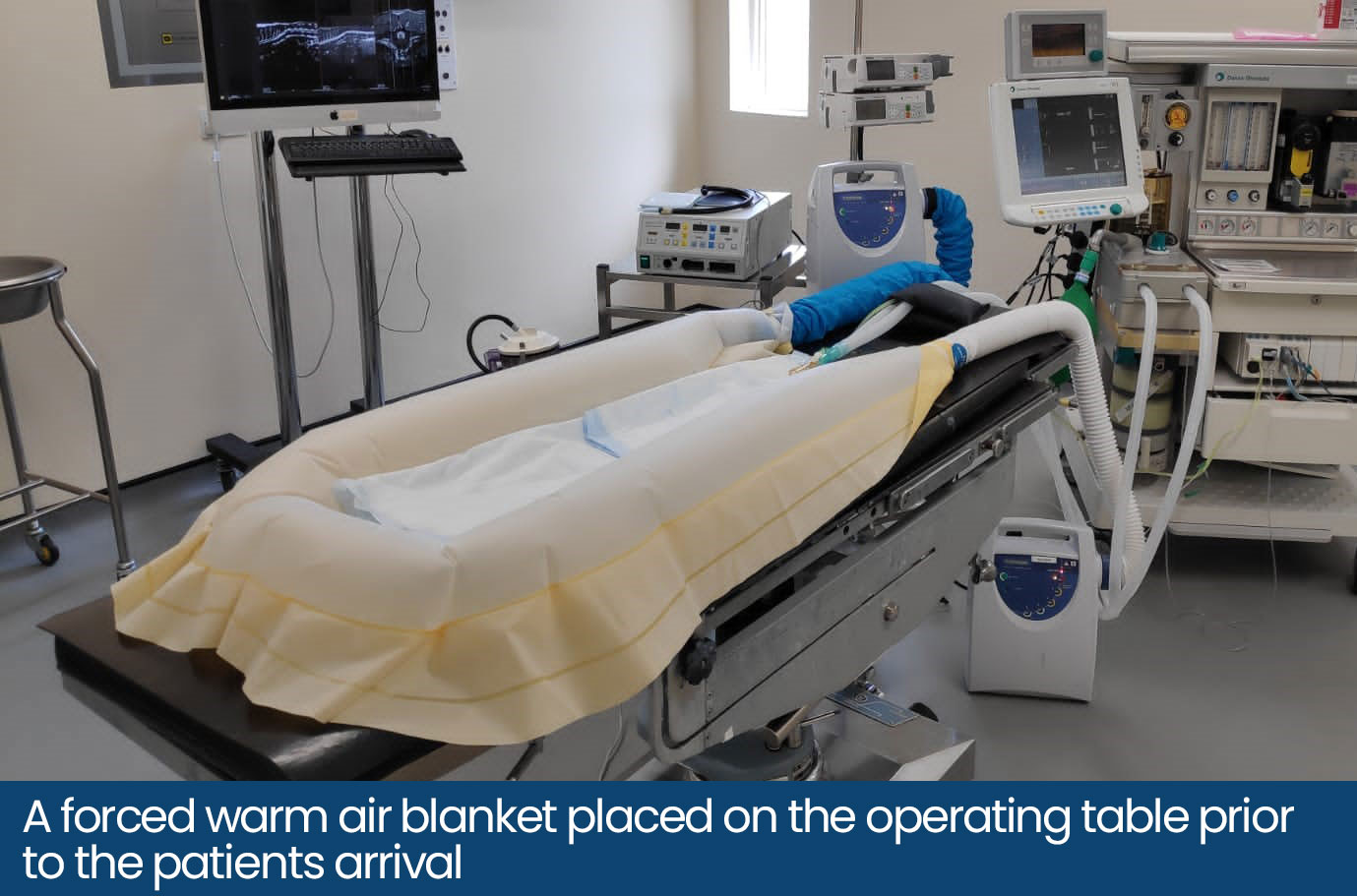
Treatment of Hypothermia:
There are two methods of warming: active and passive. In active warming, an external heat source is applied to the patient and in passive warming, measures are taken to retain the patient’s current temperature and reduce further temperature loss with the use of insulatory materials.
Common active warming techniques include the use of electrical or warm water blankets and forced warm air blankets. If warm air blankets are used, eye lubrication should be generously applied. Warming blankets should be placed on the operating table before the start of surgery as they can be difficult to place intraoperatively. Large patients may benefit from active warming methods where the external heat source is placed over them instead of under them, as their body weight can reduce the capillary blood flow in the area that is in contact with the heat source.

Warming intravenous fluids with a specialised fluid warmer to 38–39°C has a significant influence on the reduction of perioperative heat loss (Steinbacher et al., 2010).
Other methods of patient warming include warm saline enemas or bladder lavage with warmed fluids <40°C, however, this type of active-core warming is rarely performed in general practice. ‘Hot hands’ or wheat bags pose a danger to patients as their temperature may be variable, they may have hot spots or they may even rupture and are therefore not recommended by our clinical team.
In dogs, it is recommended that if a procedure lasts longer than 20 minutes, active warming techniques should be used (Clark-Price et al., 2013), and cats may require warming even sooner due to their generally smaller size.
It is important to ensure that active warming does not cause sudden vasodilation which may decrease the temperature initially and exacerbate existing hypovolaemia and hypotension. Active warming can also cause peripheral vasoconstriction where no applied heating will reach the patients core.
Passive ways to reduce temperature loss can be achieved with fabric blankets, foil blankets and insulating the extremities with baby socks or bubble wrap. Passive insulation reduces cutaneous heat loss by 30%-50% depending on how many layers are applied e.g., a blanket and surgery drapes (Mosing, 2016). To maintain body heat, it is more advantageous to warm the patient’s extremities than their trunk (Cabell et al., 1997).


If a patient has wet fur at the end of a procedure (e.g., from dental procedures or abdominal lavage), they can be dried with a hairdryer prior to recovery. The author prefers using the hairdryer on a warm setting and separating the hair with a slicker brush, taking care not to cause hot spots which may burn the patient. Expressing the patient’s bladder before surgery and at the time of recovery may also prevent them from inadvertently getting wet.
Patient Recovery and Hypothermia:
Hypothermia doesn’t just impact the peri-operative period of the patient’s anaesthesia experience; it also directly effects how that patient will recover. There is a direct link in cats from their temperature in recovery to the time of extubation, with more hypothermic patients taking longer to show control of their airway and pharyngeal tissue (Steinbacher et al., 2010).
A patient suffering from hypothermia in the post-anaesthetic period may have a prolonged recovery due to reduced drug metabolism, become bradycardic and even become hypoxaemic due to hypoventilation and shivering.
Shivering is a metabolically expensive way to perform thermogenesis as it increases oxygen consumption up to 400% and it increases glucose consumption. Therefore, a shivering patient may require oxygen supplementation. Shivering only occurs when the patient is over the thermoregulatory threshold of 35°C and usually stops between 36.5°C and 38.1°C (Steinbacher et al., 2010). Shivering can be painful, as the muscle around the surgical site contracts, and it is a sign of pain, therefore ensure adequate analgesia has been given. Shivering has been described as one of the worst aspects of recovery in human patients, so we can only assume the same for our veterinary patients.
The recovery area should be in a warm and draft-free area of the ward.
Conclusion:
Anaesthesia related hypothermia is multifactorial in its cause and correction, where prevention is easier than treatment.
Aim to start patient warming methods from premedication and monitor their temperature through to recovery, while acting on trends. After all, there isn’t anything cool about cold cats.
Burtons provide a range of warming equipment to assist you in the prevention of hypothermia. From fluid warmers to different types of warming blankets. Find out more below:
Burtons CarbonTech Heat Mat V2 now available! The latest design features all the best features from version 1, but now with 4 extra safety features, including a safe temperature regulation sensor, 3 selectable power levels, shutdown after 12 hours of use, and a disconnectable power block. Measures to 112cm L x 52cm W.
The HotDog Veterinary Warming System is unlike any other on the market, using electrically conductive fabric for even heat distribution, reducing hot spots and burn risks. The 7” touch screen controller attached provides easy control, monitoring and safety alarms. It is one of the most durable, cost-effective warming systems available. In addition to all these features, the blankets run silently and do not need to blow out any air around the surgical site. Two different controllers are available, the standard with one port for a blanket or the multi-port controller that can run 4 blankets at once. There are also 5 different blanket sizes available, these can be used under, over or folded around the patient.
Our Burtons Vet Fluid Warmer is a compact and lightweight intravenous fluid warmer, with a colourful screen for detailed information and over-temperature and low-temperature alarms. It can heat infusions from 30°C to approximately 50°C and only takes 3-5 minutes to reach the set temperature.
Acting as a barrier to all three mechanisms of heat loss: conductive, convective, and radiant, the ConRad thermal blankets have three layers made up of a fluid impermeable outer shell, an inner radiant barrier, and an inner fibrous insulating layer. These are ideal for use immediately after induction of anaesthesia to protect your patient against heat loss. These blankets are non-electrical and can be easily wiped clean after use. There are two options available, one for general use and one suitable for use during an MRI.
References
Brodbelt, D., Blissitt, K., Hammond, R., Neath, P., Young, L., Pfeiffer, D. and Wood, J., 2008. The risk of death: the Confidential Enquiry into Perioperative Small Animal Fatalities. Veterinary Anaesthesia and Analgesia, 35(5), pp.365-373.
Cabell, L., Perkowski, S., Gregor, T. and Smith, G., 1997. The Effects of Active Peripheral Skin Warming on Perioperative Hypothermia in Dogs. Veterinary Surgery, 26(2), pp.79-85.
Clark-Price, S., Dossin, O., Jones, K., Otto, A. and Weng, H., 2013. Comparison of three different methods to prevent heat loss in healthy dogs undergoing 90 minutes of general anesthesia. Veterinary Anaesthesia and Analgesia, 40(3), pp.280-284.
Clark-Price, S., 2015. Inadvertent Perianesthetic Hypothermia in Small Animal Patients. Veterinary Clinics of North America: Small Animal Practice, 45(5), pp.983-994.
Redondo, J.I., Suesta, P., Gil, L., et al., 2012a. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Veterinary Record, 170(8), pp.206–206.
Redondo, J.I., Suesta, P., Serra, I., et al., 2012b. Retrospective study of the prevalence of postanaesthetic hypothermia in dogs. Veterinary Record, 171(15), pp.374–374.
Mosing, M., Eberspächer, E., Moens, Y. and Steinbacher, R., 2010. Der Einsatz von Infusionswärmepumpen vermindert perioperative Hypothermie bei Katzen. Tierärztliche Praxis Ausgabe K: Kleintiere / Heimtiere, 38(01), pp.15-22.
Mosing, M., 2016. General principles of perioperative care. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.19-22.
Osinchuk, S., Taylor, S., Shmon, C., Parr, J. and Campbell, J., 2014. Comparison between core temperatures measured telemetrically using the CorTemp® ingestible temperature sensor and rectal temperature in healthy Labrador retrievers. The Canadian Veterinary Journal, 55(10), pp.939-945.
Posner, L., 2007. Perioperative Hypothermia in Veterinary Patients. Clinician's Brief, [online] pp.19-21. Available at: <https://files.brief.vet/migration/article/608/perioperative-hypothermia-608-article.pdf> [Accessed 14 January 2022].