Anaesthesia for the Dental Patient
February is Pet Dental Month and alongside this client-focused initiative promoting regular veterinary visits and teeth cleaning, we wanted to highlight the anaesthesia-specific considerations in patients undergoing dental treatment.
Dental disease is reported to affect 30–80% of dogs and cats that are older than 3 years. Additionally, oral diseases are some of the most common diagnoses made in small animal general practice (Snyder and Soukup, 2014).
Dental procedures can range from a quick scale and polish on a healthy dog, all the way through to multiple extractions on a geriatric cat with co-morbidities. Aside from the challenges presented when tailoring individual anaesthesia for our patients, we need to consider other challenges in dental anaesthesia such as sharing the space with the veterinary surgeon and the anaesthesia equipment, securing the airway, accidental injury from the endotracheal tube (ETT) or dental instruments, patient positioning, and pain.
In this blog, we discuss a practical approach to dental anaesthesia and then look at the range of Burtons Medical Equipment products available to help you, help your patients.
Dental Admission
Prior to the dental admission appointment, some pet owners may feel anxious about their pet requiring anaesthesia for a procedure that is often performed consciously in human medicine. The 2019 AAHA Dental Care Guidelines for Dogs and Cats stresses the importance of communication from the veterinary practice to the owner, before and after the procedure:
• Dental disease can be prevented with regular veterinary visits and home care
• If dental disease is left untreated, it can lead to chronic pain and suffering
• Regular veterinary visits can be RVN-lead in dental clinics
• Non-anaesthetic dentistry has risks and disadvantages
• Effectively address the high standard of anaesthesia and analgesia monitoring and management that is provided by your practice
• Discuss essential steps before, during and after the dental treatment. Even if pet owners are aware that extractions may be required if noted under anaesthesia, many owners would prefer another phone call to discuss and confirm this
• Animals often show signs of improved quality of life after dental treatment
The dental admission appointment should include confirmation of the patient's signalment, their medical history and the reason for dental treatment. At this time, the fasting status should be established. Fasting recommendations are variable in published literature with recent recommendations being 6–8 hours in healthy patients (Posner, 2016). However, as dental procedures are often considered ‘dirty’ procedures and performed last on the surgical list, the patient may inadvertently have a prolonged fasting period. For example, when a patient is fed at 8pm the night before anaesthesia and their procedure is performed at 1pm, this is a fasting time of 17 hours.
Prolonged fasting has been shown to increase the incidence of gastroesophageal regurgitation (GOR) and increased gastric acidity (Galatos & Raptopoulos, 1995). Feeding a small wet food meal 3 hours prior to anaesthesia did not significantly increase the gastric volume and did not reduce the gastric pH (Savvas et al., 2009). Some patients may require different fasting times or none at all e.g., paediatrics, diabetic patients.
Patient Assessment:
A physical examination should be performed prior to general anaesthesia, including an assessment of the cardiorespiratory system by auscultating both sides of the thorax. The heart rate should be a normal rate and rhythm for the patient, without pulse deficits or murmurs, and the capillary refill time should be adequate. Dental disease has been associated with conditions such as endocarditis and cardiomyopathies, likely due to the chronic inflammation connecting the bacteria in the mouth to systemic disease (Glickman et al., 2009). Bilateral lung sounds should be clear, without crackles or wheezes.
Pre-anaesthesia blood testing is performed routinely in general practice. Blood tests may not be required in patients that appear healthy if no problems were identified in the patient history or on physical examination (Alef et al., 2008). There is an association between dental disease and histological changes in the kidneys, liver and heart (Johnson and Snyder, 2015). Dental disease is often progressive in patients alongside their age and many patients may present for treatment later in their life stage. In 30-50% of dogs over 7 years old, subclinical disease was found on pre-anaesthetic blood tests (Joubert, 2007). There may be degenerative changes in organ systems, and they may have other co-morbidities. If possible, co-morbidities should be medically stabilised prior to dental procedures.
Severe dental disease raises systemic inflammatory markers such as C-reactive protein (Kouki et al., 2013). Leucocytosis is associated with inflammation or stress and may intensify following dental procedures. As thrombocytopenia is the most common bleeding disorder in veterinary medicine, platelets should be evaluated in pre-anaesthetic profiles as the consequences can be life-threatening in dental procedures (Metzger et al., 2014).
Patients with dental disease may not be eating or drinking properly and therefore, fluid and electrolyte stabilisation is important. Initial intravenous fluid therapy (IVFT) is recommended along with electrolytes testing, with supplementation via IVFT as required e.g., potassium (Johnson and Snyder, 2015). The recommended anaesthesia fluid rates are from 3ml/kg/hour in cats and 5ml/kg/hour in dogs (Grubb et al., 2020).
If a patient presents for full mouth extractions or with chronic dental disease that leaves them malnourished, they may require an oesophagostomy feeding tube prior to any dental procedures so nutrition can be optimised. Some patients may also require nutritional support and feeding tube placement postoperatively (Milella and Gurney, 2016).
Premedication:  Premedication can be administered intramuscularly (IM) or intravenously (IV). A patient that has poor body condition due to dental disease may not tolerate an IM injection (Milella and Gurney, 2016). Placement of the IV catheter in the saphenous vein is advantageous as this is further away from the bacteria-laden oral cavity and is unlikely to get wet from the dental spray.
Premedication can be administered intramuscularly (IM) or intravenously (IV). A patient that has poor body condition due to dental disease may not tolerate an IM injection (Milella and Gurney, 2016). Placement of the IV catheter in the saphenous vein is advantageous as this is further away from the bacteria-laden oral cavity and is unlikely to get wet from the dental spray.
Premedication should be individually tailored to the patient based on the ability to handle them, the procedure and their co-morbidities. The aims of premedication involve using balanced anaesthesia techniques and providing multimodal pre-emptive analgesia. Additionally, this provides sedation and anxiolysis, reduces the dose of induction drugs and contributes to a smoother recovery (Murrell, 2007).
Balanced anaesthesia is when a combination of anaesthetic drugs is used at lower doses, reducing their dose-dependent side effects e.g., opioids, alpha-2 adrenergic agonists, phenothiazines, benzodiazepines, N-methyl-D-aspartate (NMDA) antagonists.
Multimodal analgesia is when multiple classes of drugs are used to target different parts of pain processing on the nociceptive pathway (IMAGE) e.g., non-steroidal anti-inflammatory drugs (NSAIDs), opioids, NMDA antagonists, locoregional anaesthesia (nerve blocks).
Breathing Systems:
The selection of a breathing system is based on the patient size, the ability to perform positive pressure ventilation and the economic cost of its use for the intended procedure. In patients where a long anaesthesia is anticipated, a breathing system that preserves heat and humidity should be considered e.g., a circle breathing system. With the Magill breathing system, the position of the expiratory valve is at the patient end and may not be suitable for dental procedures as it increases drag. Drag is where a heavy breathing system and attachments pull on the ETT. A right-angled or flexible connector is useful to reduce drag between the breathing system and the ETT.
The patient’s recumbency is changed often during dental procedures. The breathing system must be disconnected from the ETT to prevent it from twisting in the trachea of the patient as tracheal rupture may occur, especially in cats (Mitchell et al., 2000). Clinical signs associated with tracheal ruptures include subcutaneous emphysema, coughing, gagging, dyspnoea, anorexia and pyrexia. It may be suspected immediately upon extubation, or present hours or days later.

A radiograph in a cat with subcutaneous emphysemia from a tracheal tear following dental treatment. Image credit - T.Pulchen.
To reduce exposure of anaesthetic gases to staff personnel when changing the patient’s recumbency, the vaporiser should be turned to the off position, the oxygen flow terminated, and the reservoir bag emptied into the scavenging system before disconnection from the patient. When the patient is reconnected, the oxygen flow can be re-established, and the vaporiser turned back on.
After reattaching the patient to the breathing system, the author gives several positive pressure breaths when the recumbency has changed to inflate the lung that the patient has been laying on. The author also prefers to change the patient's recumbency with a ‘legs under’ approach as any foreign content in the oral cavity easily drains away.
Most breathing systems used in veterinary medicine are made for single use in human medicine. To date, there are no official guidelines in the cleaning of disposable equipment. Cleaning with disinfectants may put the patient at risk of inhaling chemicals, causing airway irritation. Soapy water can be used to remove surface contamination (e.g., blood, dental paste).
Airway Management:
Many oral surgeries share the same considerations that dental anaesthesia does regarding airway access and management. Airway related complications increase anaesthetic related morbidity and mortality, especially in cats (Brodbelt, et al., 2007).
General anaesthesia will result in the loss of protective airway reflexes, such as coughing and swallowing, which prevent foreign material from entering the airway. The loss of these reflexes under general anaesthesia puts the patient at risk of aspiration. Foreign material during a dental procedure includes blood, water, calculus and tooth fragments. The gold standard in airway protection is with the use of an ETT with an inflated cuff (Hughes, 2016), but it is worth noting that a cuffed ETT will not prevent all liquid from tracking down the trachea (Milella and Gurney, 2016). The cuff should be checked prior to placement.
Polyvinyl chloride ETTs have a high-volume/low-pressure cuff, reducing the risk of pressure necrosis and tracheal tears. (IMAGE) The ETT should be inserted to an appropriate length, secured, excessive dead space cut from the proximal end and the cuff inflated. The ‘minimal occlusion volume’ technique is popular in practice to inflate the cuff. This technique involves one person delivering a positive pressure breath by inflating the lungs to 15-20 cmH2O and the second person inflating the cuff until no audible leak is heard (Grubb, et al., 2020).


Pharyngeal packs come in several sizes and may be placed to ensure no foreign material enters the airway. If pharyngeal packs are used, it is useful to make a note on the anaesthetic monitoring form to ensure that they are removed prior to recovery. Additionally, it can be tied to the ETT so that it is pulled out at the same time during extubation if it is accidentally forgotten.
Use of mouth gags in cats:
Spring-loaded gags generate constant force and excessive mouth opening in cats. This causes the bulging of soft tissues between the mandible and the tympanic bulla, compressing the maxillary arteries between the bony structures of the head (Martin-Flores et al., 2014). With compromised blood flow to the maxillary arteries, severe neurological deficits and blindness can occur, which may be compounded by anaesthesia related hypotension (Milella and Gurney, 2016). Therefore, spring gags shouldn’t be used in cats (Grubb and Lobprise, 2020b).
In an average-sized cat, mouth gags that opened the mouth more than 42mm between the canine teeth produced blood flow deficits. A 42mm gag is the equivalent of the size of a needle cap. Blood flow deficits were not observed when the mouth was opened 20-30mm (Martin-Flores et al., 2014).
There have been reports of permanent post-anaesthetic bilateral deafness in both cats and dogs after dental (and ear cleaning) procedures. Older animals were more susceptible to deafness, however, there are no other identifiable causes as to why this occurs. The prevalence of post-anaesthetic deafness is low (Stevens–Sparks & Strain, 2010).
Monitoring Considerations:
During dental procedures, the patient is moved more often than with other surgeries. The head is manipulated to visualise and gain access to parts of the mouth and the patient recumbency is changed frequently. Excessive head manipulation can irritate and traumatise the larynx and trachea, and laryngeal oedema can occur in cats. Care should be taken to stabilise the head when force is being applied in the mouth, usually during extraction of the teeth.
changed frequently. Excessive head manipulation can irritate and traumatise the larynx and trachea, and laryngeal oedema can occur in cats. Care should be taken to stabilise the head when force is being applied in the mouth, usually during extraction of the teeth.
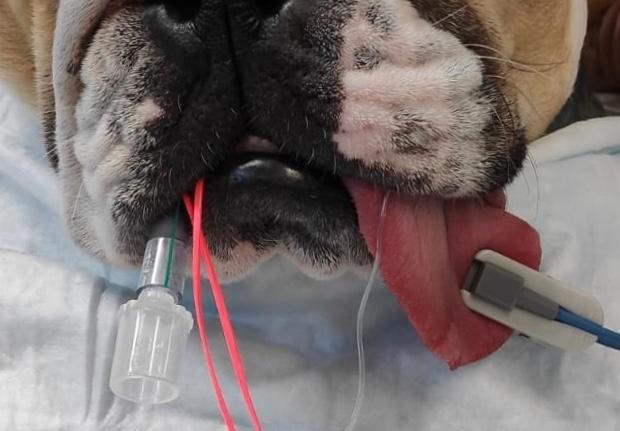
Another predicament with anaesthesia and dental procedures is that the space is shared between the anaesthetist and the surgeon, affecting airway access and some monitoring techniques. The Association of Veterinary Anaesthesia’s guidelines for safer anaesthesia recommends the use of pulse oximetry, capnography and blood pressure along with the standard ‘hands on’ monitoring. Some considerations for the use of monitoring equipment includes: • Obtaining reliable pulse oximetry readings can be challenging when the probe is placed on the tongue. Alternatively, try other pigment-free areas such as the prepuce, vulva, toe webbing or pinna. Rectal and reflectance probes are also available.
• Obtaining reliable pulse oximetry readings can be challenging when the probe is placed on the tongue. Alternatively, try other pigment-free areas such as the prepuce, vulva, toe webbing or pinna. Rectal and reflectance probes are also available.
• Capnography is beneficial when performing dental procedures as any obstructions within the ETT can be identified. If there is a kink in the ETT due to positioning or if accidental disconnection of the breathing system occurs, this will be shown on the capnograph as a shark fin trace or a sudden loss of waveform (IMAGE). Mainstream analysers can be bulky, are easily damaged when moved and may malfunction with water vapour. Side stream sampling improves access to the patient's mouth and reduces drag from the breathing system. The bulk of side stream connectors and dead space can be reduced with the use of a low dead space connector (ETT <5.0mm).
• When obtaining blood pressure readings during anaesthesia, there is access to the limbs and the tail. Conveniently, patients are often laying on their side, reducing inaccurate readings where the limb is at a different height to the right atrium of the heart.
There has been one reported incidence of a fatal air embolism after the use of a high-speed, air-driven, water-cooled dental drill (Gunew et al., 2008). There are reports in human literature, but this appears the only one in veterinary medicine. Air embolisms cause acute hypotension, loss of capnography waveform, and cardiac and respiratory arrest. Cardiopulmonary resuscitation should be performed if a cardiopulmonary arrest has occurred.
Ocular lubrication should be applied frequently during anaesthesia as the patient loses the ability to blink at a surgical plane of anaesthesia, and there is a reduction in tear production associated with many anaesthetic drugs. Generous ocular lubrication will also create a barrier from dental spray and debris from the drill.
Alongside recording monitoring of the anaesthesia, the RVN can complete a medico-legal record of dental pathology and treatment.
Hypothermia:
Hypothermia is a common complication in patients during the anaesthesia period. Patients requiring anaesthesia for dental treatment often have procedures that are long in duration, there is frequent repositing of the patient, and they have a constant stream of cold water running over their mouth during scaling, drilling and rinsing. This often means that the patient recovers from anaesthesia with very wet fur on their head which should be dried prior to recovery. The author prefers using the hairdryer on a warm setting and separating the hair with a slicker brush, taking care not to cause hot spots which may burn the patient and taking care around the eyes with the brush. Using an absorbable barrier such as an incontinence pad with a hole in the middle for the nose and mouth to come through can prevent the patient from getting excessively wet.
Thankfully, as dental procedures are contained to the head, the anaesthetist has complete access to the rest of the patient. Active warming equipment, such as heat mats, can be used while the patient's head is positioned over a drain. Passive warming can be applied to the patient with fabric blankets, foil blankets and by insulating the extremities with baby socks or bubble wrap.
As dental procedures can be long in duration, the dental equipment should be checked prior to its use to ensure everything is in working order e.g., sufficient water supply, air compressing equipment functions. This will avoid any unnecessary troubleshooting while the patient is anaesthetised.
Aim to start patient warming methods from premedication and monitor their temperature through to recovery, while acting on trends.
Pain:
Animals display obvious behaviours when in acute pain such as crying, guarding the painful area or limping. Animals with excruciating chronic dental pain will often suffer in silence, with subtle behaviours such as inappetence, posture change, drooling, becoming head shy and pawing at their face. However, some animals may also be stoic and hide their reaction to pain. Chronic pain and inflammation can negatively impact an animal’s quality of life.
While periodontal disease doesn’t impact the metabolism of anaesthesia drugs, chronic pain and inflammatory states can cause changes in the central or peripheral nervous system, resulting in ‘wind up’ pain. Central sensitisation occurs when the spinal cord is subjected to repeated and uncontrolled painful stimuli causing excitatory neuropeptides, including substance P and glutamate, to be released. These bind to and stimulate the NMDA receptor, causing painful stimuli to be amplified. An NMDA antagonist such as ketamine can be administered to block substance P and glutamate from binding to this receptor. While the patient has altered pain processing, other drugs such as opioids and alpha-2 adrenergic agonists can become ineffective. Inflammation can also impact the effectiveness of local anaesthetic drugs (Snyder and Soukup, 2014), which are discussed below.
Almost all dental procedures that involve anything more than a scale and polish are likely to be painful. The benefits of effective pain management during anaesthesia include the ability to reduce the minimal alveolar concentration (MAC) of inhalant agents, reducing their dose-dependent side effects. If the heart rate, blood pressure and/or respiratory rate increases in response to surgical stimuli during anaesthesia, then additional intraoperative analgesia may be required. In some long dental procedures, the initial analgesic provided in the premedication may require a repeat dose e.g., methadone has a duration of action of 4-8 hours, but some patients may require analgesia from 4 hours (Kerr, 2016). Postoperative pain management promotes a smoother recovery period, patient comfort and welfare.
Multimodal analgesia should be provided when pain is anticipated. An opioid in combination with an NSAID is commonly administered to patients who are having dental procedures:
• Full mu-opioids are ideal analgesics for dental procedures as they can be titrated, are synergistic with sedatives, they have an antagonist effect at the NMDA receptor and have a faster onset than buprenorphine, a partial mu agonist. Butorphanol is a kappa agonist and mu antagonist and does not provide adequate analgesia in dental procedures as its effects are short-lived. Because of its lesser efficacy and short duration of action, butorphanol is not recommended as an effective analgesic for dental procedures (Mills, 2016).
• NSAIDs prevent the formation of prostaglandins by targeting the cyclooxygenase enzyme responsible for prostaglandin production.
Constant rate infusions of opioids or NMDA antagonists can be considered. They offer stable and effective analgesia in comparison to IM or IV administration of analgesia as they avoid “peaks and troughs” of drug plasma concentrations.
Adjuvant analgesics such as ketamine, gabapentin and paracetamol can be administered, if suitable. It is important to remember that ketamine preserves the laryngeal and pharyngeal reflexes, so the patient may appear to be swallowing while under anaesthesia.
A multidimensional composite pain scale should be used postoperatively, however, none are validated for dental or oral pain in small animals. The Feline Grimace Scale has been shown to be a reliable tool for pain assessment in cats undergoing dental extractions (Watanabe et al., 2020). If there is any doubt that the patient is experiencing pain, analgesia should be given, and the response assessed.
Locoregional anaesthesia:
Utilising locoregional anaesthesia techniques contributes to a balanced anaesthesia protocol and a multimodal analgesia plan. Local anaesthetic drugs prevent the transmission of painful stimuli from reaching the central nervous system. The use of locoregional anaesthesia improves anaesthetic safety because the inhalant dose or MAC is decreased (Grubb and Lobprise, 2020a) and it can result in lower postoperative pain scores compared to patients that do not receive one (Aguiar et al., 2014).
It is important to consider that when local nerve blocks are performed for surgical extraction of teeth, they are less effective in regions of tissue infection (Grubb and Lobprise, 2020b). This is due to local inflammation of the tissue, which changes the tissue pH, reducing the drug's efficacy and effect (Snyder and Soukup, 2014). As some nerves may have multiple branches that are potentially not desensitised, local nerve blocks should not be relied upon as the only analgesia for dental surgery.
Different local anaesthetic drugs can be used depending on practice preference and the needs of the patient. Drugs include lidocaine, bupivacaine, mepivacaine and ropivacaine. While these different drugs have published durations of action, they may have prolonged action when injected in the dental foramen, with bupivacaine being reported to exceed 24 hours in one study (Snyder et al., 2016). Drugs can be added to the local anaesthetic to prolong its duration of action, including buprenorphine and adrenaline (Snyder et al., 2016 (Lanz, 2003).
Doses and toxicity should be considered for all individual local anaesthetics, especially as patients may need multiple nerve blocks in a single treatment. There are recommended volumes of infiltration (Lantz, 2003):
• Cats 0.2–0.25ml per site
• Dogs 0.25, 0.5, or 1ml per site for small, medium and large dogs, respectively
Accurate and detailed knowledge of the neuroanatomy of the oral cavity is necessary when preparing to perform local nerve blocks, especially as some blood vessels run alongside the nerve. Prior to injecting the local anaesthetic drug, the syringe should be aspirated to ensure intravascular injection does not occur. Intravascular injection and nerve damage are common complications with locoregional techniques. Listed below are common nerve blocks that can be performed in dental and oral surgery:
• Mental nerve block (Figure 1)– This nerve block desensitises the teeth rostral to the mental foramen if the needle is advanced caudally into the canal, which can be difficult to achieve in cats and small dogs, otherwise only the buccal soft tissues and lower lip are desensitised.
• Mandibular nerve block (Figure 2) – This nerve block desensitises the mandibular teeth rostral to the block and the skin over the lower lip. It can be performed intra- or extraoral.
• Maxillary nerve block (Figure 3) – This nerve block captures the maxillary nerve before it enters the infraorbital canal and desensitises the upper teeth and lip, nose, maxilla and hard and soft palates. It can be performed intra- or extraoral.
• Infraorbital nerve block (Figure 4) – this nerve is where the maxillary nerve exits the infraorbital canal. Depositing the local anaesthetic drug at the foramen does not provide dental analgesia as it is a sensory nerve to the nose and upper lip. To desensitise teeth with this block, the needle must be inserted into the infraorbital canal, which may cause nerve damage with poor technique.

When performing an infraorbital nerve block, care should be taken regarding accidental globe penetration in cats and brachycephalic breeds, who have a short infraorbital canal. Globe penetration can be avoided by not advancing a needle further than 2–3 mm into the canal and directing the needle ventrally not dorsally towards the globe. Accidental globe penetration can also occur in the intraoral approach to the maxillary nerve block in cats (Perry et al., 2014).
Accident globe penetration in a brachycephalic
When performing a local nerve block, the patient may respond due to the acidic nature of the local anaesthetic, causing an increase in the heart rate, respiratory rate and even patient movement. This can confirm that the drug has been administered to the correct location. However, if the patient does not react, it does not correlate to the local anaesthetic being placed in the wrong location as the patient’s anaesthesia depth may impact this (Snyder and Soukup, 2014).
When administering local anaesthetics through a small gauge needle, administer the volume slowly as exerting pressure at a fast velocity can cause tissue damage (Snyder and Soukup, 2014).
Self-trauma due to inadvertent anaesthesia of the tongue is anecdotally mentioned in the veterinary literature, however, to the author's knowledge, has not been reported or published in scientific journals. This occurs when the local anaesthetic drug is deposited too far medial and caudally performing a mandibular nerve block (which has 3 branches), and the lingual and mylohyoid branches of the mandibular nerve are desensitised instead of the inferior alveolar nerve (Milella and Gurney, 2016).
An RVN can perform oral local nerve blocks under the supervision of a veterinary surgeon, however, they are unable to perform dental extractions that require the use of dental instruments.
Recovery:
The postoperative recovery period is a high-risk period; 47% of dogs and 61% of cat fatalities occurred during this time and half of them within the first 3 hours. Respiratory complications were identified as a common cause. The CEPSAF study concluded that greater patient care is needed in the recovery period, and this can easily be managed with continued patient monitoring and observation (Brodbelt, et al., 2008).
Prior to recovery, the oral cavity should be checked with a laryngoscope or other light source to ensure there is no debris that could be aspirated after extubation. It may be beneficial to position the patient with their nose facing down so that any foreign material can drain from the mouth. In dogs, the ETT should be removed once there is a strong swallowing reflex. The ETT can be left partially inflated for extubation as this will pull forward any foreign material that may have accidentally made its way into the airway. In cats, extubation should be performed with the cuff deflated where there is a strong pinna reflex (ear twitch) or if they have a strong palpebral blink.
Cats have a strong laryngeal reflex, causing immediate closure of the glottis and vocal cords. Airway obstruction can occur in cats in the recovery period due to laryngospasm and oedema and is common after dental procedures. A laryngospasm is usually self-limiting and can be managed by extending the head and neck forward while providing flow-by oxygen. If there is a sustained laryngospasm causing cyanosis or oxygen saturation of <90%, the patient may need to re-anaesthetised, and the larynx desensitised again.
Inadequately controlled pain in the recovery period is considered a postoperative complication and must be managed appropriately (Mosing, 2016). Pain scoring with a validated system should be performed every 30 minutes during the recovery period, before and after analgesia is administered (Kata et. al., 2015). When performing a pain score in the recovery period, it is important to factor in if the patient is too sedated to respond appropriately, as sedation does not equal adequate analgesia.
Conclusion:
There are specific considerations for patients undergoing dental procedures that are potentially overlooked as they may be perceived differently from other surgical procedures, such as soft tissue and orthopaedic surgeries.
It is also important to highlight that the benefits of any dental procedures performed under general anaesthesia can be short-lived unless there is effective home care. This can be promoted through RVN-lead dental clinics, providing client education and demonstrations of tooth cleaning. This straightforward and inexpensive nursing care promotes regular veterinary visits, which strengthens the practice bond with the client.
Burtons Veterinary Equipment Key Dental Anaesthesia Products:
Burtons Veterinary Equipment manufacture and provide a large range of dental and anaesthesia equipment with some of the key machines being industry famous. Think VETair dental machines and the Burtons Adaptable anaesthesia machine. Below are just a few of the products that are linked to this article. You can view the complete range here: Anaesthesia Category / Dental Category
The Burtons VETair Classic retains all the key industry-leading features of its predecessor the original VETair, but now comes with an in-built oil-free compressor, lowering lifetime maintenance cost by removing the need for oil changes. It has been designed to incorporate the same technology as Burtons large oxygen concentrators, a clever combination of spring-mounted components and delay timer tech softens the inertia of starting and stopping the pump, irradicating the well-known issue of noisy, oil free compressors and leaving you with a gentle and quiet environment for stress-free dental procedures.
The VETair classic still boasts its robust, easy-to-clean Burtons Lifetime stainless steel frame with its three-station control unit for high and low speed hand pieces, as well as a three-way syringe for your water/air/mist. It is also surprisingly compact, with direct connection to your dental scaler thanks to its onboard water supply, concealed internal storage drawer, cable tidy’s and four-way power socket for all your accessories attached. When not in use, the fully mobile unit can be neatly stored under your knee space tub table and secured by its lockable wheels.
Our Low Dead Space Adapters allow a side stream capnography line to be directly attached, reducing apparatus dead space.
Although often required, attachments used between a patient and a breathing system can lengthen the apparatus dead space and contribute to the rebreathing of expired gases, especially when the fresh gas flow is not adequate. Heat and moisture exchangers can also increase this.
Burtons CarbonTech Heat Mats feature a new generation of technology that is specifically designed for even heat distribution. This works by delivering a low contact heat temperature whilst directly transferring the energy necessary for warming the patient, meaning no heat is stored within the blanket and therefore reducing the risk of hot spots and burns. The material used ensures ultimate comfort for the patient, as well as allowing some flexibility in how they are used. The target temperature is reached within just 5 minutes of switching on. These mats are available in two sizes, ideal for operating tables and patient kennels.
References
Aguiar, J. et al., 2014. Analgesic effects of maxillary and inferior alveolar nerve blocks in cats undergoing dental extractions. Journal of Feline Medicine and Surgery, 17(2), pp.110–116.
Alef, M., Von Praun, F. & Oechtering, G., 2008. Is routine pre-anaesthetic haematological and biochemical screening justified in dogs? Veterinary Anaesthesia and Analgesia, 35(2), pp.132–140.
Brodbelt, D.C. et al., 2007. Risk factors for anaesthetic-related death in cats: Results from the Confidential Enquiry into Perioperative Small Animal Fatalities (CEPSAF). British Journal of Anaesthesia, 99(5), pp.617–623.
Brodbelt, D., Blissitt, K., Hammond, R., Neath, P., Young, L., Pfeiffer, D. and Wood, J., 2008. The risk of death: the Confidential Enquiry into Perioperative Small Animal Fatalities. Veterinary Anaesthesia and Analgesia, 35(5), pp.365-373.
Galatos, A. & Raptopoulos, D., 1995. Gastro-oesophageal reflux during anaesthesia in the dog: The effect of preoperative fasting and premedication. Veterinary Record, 137(19), pp.479–483.
Glickman, L.T. et al., 2009. Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs. Journal of the American Veterinary Medical Association, 234(4), pp.486–494.
Gunew, M., Marshall, R., Lui, M. and Astley, C., 2008. Fatal venous air embolism in a cat undergoing dental extractions. Journal of Small Animal Practice, 49(11), pp.601-604.
Grubb, T. and Lobprise, H., 2020a. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Veterinary Medicine and Science, 6(2), pp.209-217.
Grubb, T. and Lobprise, H., 2020b. Local and regional anaesthesia in dogs and cats: Descriptions of specific local and regional techniques (Part 2). Veterinary Medicine and Science, 6(2), pp.218-234.
Grubb, T. et al., 2020. 2020 AAHA anesthesia and monitoring guidelines for dogs and cats. Journal of the American Animal Hospital Association, 56(2), pp.59–82.
Hughes L., 2016. Breathing systems and ancillary equipment. In: Duke-Novakovski T, de Vries M and Seymour C., eds. BSAVA manual of canine and feline anaesthesia and analgesia. 3rd ed. Gloucester: British Small Animal Veterinary Association; pp 58–59.
Joubert, K.E., 2007. Pre-anaesthetic screening of Geriatric Dogs. Journal of the South African Veterinary Association, 78(1), pp.31–35.
Kata, C., Rowland, S. and Goldberg, M., 2015. Pain Recognition in Companion Species, Horses, and Livestock. In: M. Goldberg and N. Shaffran, ed., Pain Management for Veterinary Technicians and Nurses. Iowa: John Wiley & Sons, Inc, pp.15-29.
Kerr, C., 2016. Pain management I: systemic analgesics. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.125.
Lantz, G., 2003. Regional Anesthesia for Dentistry and Oral Surgery. Journal of Veterinary Dentistry, 20(3), pp.181-186.
Martin-Flores, M., Scrivani, P., Loew, E., Gleed, C. and Ludders, J., 2014. Maximal and submaximal mouth opening with mouth gags in cats: Implications for maxillary artery blood flow. The Veterinary Journal, 200(1), pp.60-64.
Milella, L., Gurney, M., 2016. Dental and Oral Surgery. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.276-277.
Metzger, F., DeNicola, D. and Barteaux, W., 2014. Help avoid anesthetic complications—run a complete blood count on every preanesthetic patient. [online] Idexx. Available at: <https://www.idexx.co.uk/files/run-cbc-avoid-anesthetic-complications.pdf> [Accessed 25 January 2022].
Mills, A., 2016. Pain management for veterinary dental patients. Today's Veterinary Nurse. Available at: https://todaysveterinarynurse.com/articles/pain-management-for-dental-patients/ [Accessed January 26, 2022].
Mitchell, S.L. et al., 2000. Tracheal rupture associated with intubation in cats: 20 cases (1996–1998). Journal of the American Veterinary Medical Association, 216(10), pp.1592–1595.
Murrell, J., 2007. Choice of premedicants in cats and dogs. In Practice, 29(2), pp.100–106.
Perry, R., Moore, D. and Scurrell, E., 2014. Globe penetration in a cat following maxillary nerve block for dental surgery. Journal of Feline Medicine and Surgery, 17(1), pp.66-72.
Posner, L., 2016. Pre-anaesthetic assessment and preparation. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.6-12.
Savvas, I., Rallis, T. & Raptopoulos, D., 2009. The effect of pre-anaesthetic fasting time and type of food on gastric content volume and acidity in dogs. Veterinary Anaesthesia and Analgesia, 36(6), pp.539–546.
Stevens–Sparks, C.K. & Strain, G.M., 2010. Post–anesthesia deafness in dogs and cats following dental and ear cleaning procedures. Veterinary Anaesthesia and Analgesia, 37(4), pp.347–351.
Snyder, L.B., Snyder, C.J. & Hetzel, S., 2016. Effects of buprenorphine added to bupivacaine infraorbital nerve blocks on isoflurane minimum alveolar concentration using a model for acute dental/oral surgical pain in dogs. Journal of Veterinary Dentistry, 33(2), pp.90–96.
Snyder, C. and Soukup, J., 2014. Oral and maxillofacial disorders. In: L. Snyder and R. Johnson, ed., Canine and Feline Anesthesia and Co‐Existing Disease, 1st ed. New Jersey: John Wiley & Sons, Inc.
Watanabe, R. et al., 2020. Inter-rater reliability of the feline grimace scale in cats undergoing dental extractions. Frontiers in Veterinary Science, 7.
Aguiar, J. et al., 2014. Analgesic effects of maxillary and inferior alveolar nerve blocks in cats undergoing dental extractions. Journal of Feline Medicine and Surgery, 17(2), pp.110–116.
Alef, M., Von Praun, F. & Oechtering, G., 2008. Is routine pre-anaesthetic haematological and biochemical screening justified in dogs? Veterinary Anaesthesia and Analgesia, 35(2), pp.132–140.
Brodbelt, D.C. et al., 2007. Risk factors for anaesthetic-related death in cats: Results from the Confidential Enquiry into Perioperative Small Animal Fatalities (CEPSAF). British Journal of Anaesthesia, 99(5), pp.617–623.
Brodbelt, D., Blissitt, K., Hammond, R., Neath, P., Young, L., Pfeiffer, D. and Wood, J., 2008. The risk of death: the Confidential Enquiry into Perioperative Small Animal Fatalities. Veterinary Anaesthesia and Analgesia, 35(5), pp.365-373.
Galatos, A. & Raptopoulos, D., 1995. Gastro-oesophageal reflux during anaesthesia in the dog: The effect of preoperative fasting and premedication. Veterinary Record, 137(19), pp.479–483.
Glickman, L.T. et al., 2009. Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs. Journal of the American Veterinary Medical Association, 234(4), pp.486–494.
Gunew, M., Marshall, R., Lui, M. and Astley, C., 2008. Fatal venous air embolism in a cat undergoing dental extractions. Journal of Small Animal Practice, 49(11), pp.601-604.
Grubb, T. and Lobprise, H., 2020a. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Veterinary Medicine and Science, 6(2), pp.209-217.
Grubb, T. and Lobprise, H., 2020b. Local and regional anaesthesia in dogs and cats: Descriptions of specific local and regional techniques (Part 2). Veterinary Medicine and Science, 6(2), pp.218-234.
Grubb, T. et al., 2020. 2020 AAHA anesthesia and monitoring guidelines for dogs and cats. Journal of the American Animal Hospital Association, 56(2), pp.59–82.
Hughes L., 2016. Breathing systems and ancillary equipment. In: Duke-Novakovski T, de Vries M and Seymour C., eds. BSAVA manual of canine and feline anaesthesia and analgesia. 3rd ed. Gloucester: British Small Animal Veterinary Association; pp 58–59.
Joubert, K.E., 2007. Pre-anaesthetic screening of Geriatric Dogs. Journal of the South African Veterinary Association, 78(1), pp.31–35.
Kata, C., Rowland, S. and Goldberg, M., 2015. Pain Recognition in Companion Species, Horses, and Livestock. In: M. Goldberg and N. Shaffran, ed., Pain Management for Veterinary Technicians and Nurses. Iowa: John Wiley & Sons, Inc, pp.15-29.
Kerr, C., 2016. Pain management I: systemic analgesics. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.125.
Lantz, G., 2003. Regional Anesthesia for Dentistry and Oral Surgery. Journal of Veterinary Dentistry, 20(3), pp.181-186.
Martin-Flores, M., Scrivani, P., Loew, E., Gleed, C. and Ludders, J., 2014. Maximal and submaximal mouth opening with mouth gags in cats: Implications for maxillary artery blood flow. The Veterinary Journal, 200(1), pp.60-64.
Milella, L., Gurney, M., 2016. Dental and Oral Surgery. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.276-277.
Metzger, F., DeNicola, D. and Barteaux, W., 2014. Help avoid anesthetic complications—run a complete blood count on every preanesthetic patient. [online] Idexx. Available at: <https://www.idexx.co.uk/files/run-cbc-avoid-anesthetic-complications.pdf> [Accessed 25 January 2022].
Mills, A., 2016. Pain management for veterinary dental patients. Today's Veterinary Nurse. Available at: https://todaysveterinarynurse.com/articles/pain-management-for-dental-patients/ [Accessed January 26, 2022].
Mitchell, S.L. et al., 2000. Tracheal rupture associated with intubation in cats: 20 cases (1996–1998). Journal of the American Veterinary Medical Association, 216(10), pp.1592–1595.
Murrell, J., 2007. Choice of premedicants in cats and dogs. In Practice, 29(2), pp.100–106.
Perry, R., Moore, D. and Scurrell, E., 2014. Globe penetration in a cat following maxillary nerve block for dental surgery. Journal of Feline Medicine and Surgery, 17(1), pp.66-72.
Posner, L., 2016. Pre-anaesthetic assessment and preparation. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia, 3rd ed. Gloucester: British Small Animal Veterinary Association, pp.6-12.
Savvas, I., Rallis, T. & Raptopoulos, D., 2009. The effect of pre-anaesthetic fasting time and type of food on gastric content volume and acidity in dogs. Veterinary Anaesthesia and Analgesia, 36(6), pp.539–546.
Stevens–Sparks, C.K. & Strain, G.M., 2010. Post–anesthesia deafness in dogs and cats following dental and ear cleaning procedures. Veterinary Anaesthesia and Analgesia, 37(4), pp.347–351.
Snyder, L.B., Snyder, C.J. & Hetzel, S., 2016. Effects of buprenorphine added to bupivacaine infraorbital nerve blocks on isoflurane minimum alveolar concentration using a model for acute dental/oral surgical pain in dogs. Journal of Veterinary Dentistry, 33(2), pp.90–96.
Snyder, C. and Soukup, J., 2014. Oral and maxillofacial disorders. In: L. Snyder and R. Johnson, ed., Canine and Feline Anesthesia and Co‐Existing Disease, 1st ed. New Jersey: John Wiley & Sons, Inc.
Watanabe, R. et al., 2020. Inter-rater reliability of the feline grimace scale in cats undergoing dental extractions. Frontiers in Veterinary Science, 7.