Anaesthesia for the Laparoscopic Patient
Laparoscopic surgery is becoming more common in veterinary medicine for non-invasive procedures including ovariectomies, ovariohysterectomies and liver biopsies. This minimally invasive approach to surgery has many appeals including a lower risk of postoperative wound breakdown compared to patients with an open laparotomy surgical approach (Charlesworth and Sanchez, 2019), reduced tissue trauma, smaller incision size and decreased handling of the gastrointestinal tract which leads to improved postoperative comfort and recovery times (de Rezende and Mama, 2015).
From an anaesthetic perspective, there is a significant reduction in postoperative pain scores after an ovariohysterectomy (Hancock et al., 2005, Devitt et al., 2005), and an increase in postoperative activity levels in patients that had an ovariectomy compared to those who had a midline laparotomy approach (Culp et al., 2009). There is also a decrease in the stress response markers serum cortisol and glucose postoperatively in patients with a laparoscopic ovariectomy compared to those with an open laparotomy (Devitt et al., 2005). A decrease in inflammatory mediators such as C-reactive proteins, interleukin-6 and circulating leukocytes is also seen in patients undergoing laparoscopic surgeries compared to an open surgical procedure (de Rezende and Mama, 2015).
A laparoscopic approach should not be thought of as “routine” or of a lower risk than other types of surgical anaesthesia, as a patient’s American Society of Anesthesiologists (ASA) grade will still predict the patient’s anaesthetic risk based on their physiological status (Hayden and Cowman, 2011). Some patients with pre-existing cardio- or pulmonary disease (e.g. valvular heart disease, congestive heart failure) have a higher risk for complications when having a laparoscopic procedure due to the haemodynamic and ventilatory changes that occur with abdominal insufflation (de Rezende and Mama, 2015).
Whilst the procedure type and patient physiological status (e.g. co-morbidities) will dictate the anaesthesia, analgesia plan and protocol, there are specific challenges relating to the anaesthesia in the laparoscopic patient due to the physiological impact that insufflating the abdomen has, which the veterinary team should be familiar with and are discussed throughout this article.
Laparoscopic Equipment and Positioning
A laparoscopic set-up is standard, with two to three incisions made and ports placed, into the abdomen:
- One for insufflation gases to inflate the abdomen
- One for the laparoscopic camera
- One for the surgical instruments

Laparoscopic Equipment
Carbon dioxide (CO2) is the insufflator gas of choice as it is inert, very soluble in blood and isn’t combustible, which is advantageous when performing surgery with electrosurgery. Other gases such as nitrous oxide, atmospheric air, oxygen, nitrogen and helium can be used to produce pneumoperitoneum, however, CO2 is by far the most popular (Fukushima et al., 2011). The abdomen is typically insufflated to a pressure of 10-15mmHg (13-20cmH2O), allowing the visualisation of abdominal viscera.
It is important to note that CO2 is compressed in cylinders as a liquid, so it must be delivered to the equipment and patient from an upright position.
Organ puncture can occur with the introduction of laparoscopic equipment and requires emergent intervention by both the anaesthetist and surgeon, with potential conversion to an open surgical technique required (de Rezende and Mama, 2015).
Patient Positioning
The patient may be positioned at an angle of 10-15° in the Trendelenburg position (the head lower than the abdomen), or a reversed Trendelenburg position (head higher than the abdomen) or laying in dorsal recumbency but tilted from side to side into a 45° oblique recumbency.
A reversed Trendelenburg position may minimise the effect of insufflation on ventilation and oxygenation (Perilli et al., 2000), however, the positioning is usually dictated by the surgeon and the procedure being performed.

A laparoscopic ovariectomy.
Considerations
To decrease any negative influences that positioning, and the pneumoperitoneum have during the patient’s anaesthesia, such as hypotension, pneumothorax, air embolism and haemorrhage, the intrabdominal pressure (IAP) should not exceed 15mmHg (20cmH2O) (Doerfelt and Eberspacher-Schweda, 2010).
Regardless of the IAP, multiple physiological processes are affected by laparoscopic surgery to some degree, and their specific anaesthetic considerations are discussed below.
Cardiac Output and Blood Pressure
Haemodynamic changes may be caused by the direct effect of CO2 on the cardiovascular system and also indirectly through its sympathetic stimulation causing tachycardia, hypertension and cardiac arrhythmias (Fukushima et al., 2011; Henny and Hofland, 2005).
Most haemodynamic changes occur in the first 15 minutes of insufflation, which may be a result of either hypercapnia and/or distension of the abdomen due to the pneumoperitoneum. The biggest cardiopulmonary change is associated with the time of the initial insufflation and then again at the time of deflation, caused by the sudden changes in IAPs. This should be performed slowly to allow accommodation of these pressure changes and compensation by the patient.
A reduction in cardiac output may occur due to the compression of the caudal vena cava, reducing venous return to the heart, but keeping the IAP <15mmHg (20cmH2O) will reduce the degree of this venous compression. Aortic compression causing an increase in system vascular resistance can also occur with increasing IAP (Hayden and Cowman, 2011). In response to a decrease in cardiac output, the heart rate may be seen to increase and the patient may actually have a normal blood pressure, assisted by the increase in systemic vascular resistance and the release of plasma catecholamines (de Rezende and Mama, 2015).

An IAP of 13mmHg during laparoscopic surgery.
Distention of the peritoneum due to abdominal insufflation may increase vagal tone, which can lead to bradyarrhythmias that could cause a cardiac arrest if not treated immediately (de Rezende and Mama, 2015). Management and treatment include decreasing the IAP and potentially administering an anticholinergic drug such as atropine.
Significant haemorrhage during laparoscopic surgery is one of the contributing factors to conversion into an open surgical approach (Buote et al., 2010), so the patient should be clipped appropriately and aseptically prepared before the laparoscopic procedure. It is important to note that the degree of intra-operative bleeding may not be immediately noticeable as there is venous compression with insufflation, and it may not be noticed until the abdomen is deflated for recovery (de Rezende and Mama, 2015).
Ventilation
There is both a chemical and mechanical impact on ventilation related to the pneumoperitoneum required for abdominal insufflation.
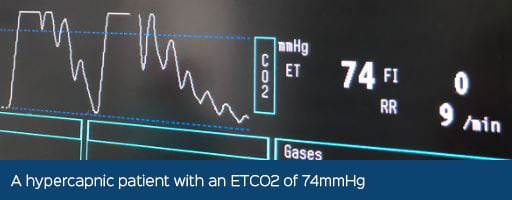
The CO2 used to insufflate the abdomen can increase end-tidal CO2 (ETCO2) values as the gas diffuses into the splanchnic circulation (Schauvliege, 2016). The impact on ventilation appears to be lessened than with other inert gases such as nitrous oxide or helium, however, they have other disadvantages which are beyond the scope of this article (de Rezende and Mama, 2015).
An increase in CO2 levels in the body can decrease the blood pH, known as an acidaemia, which can affect normal cellular function and cause an increase in sympathetic tone, which may predispose the patient to cardiac arrhythmias such as tachycardia and ventricular premature contractions.
Most healthy patients will tolerate an elevated ETCO2, it should be maintained below 55mmHg (de Rezende and Mama, 2015). In the conscious patient, a response to an increase in ETCO2 would result in them increasing their minute volume (MV) by increasing their respiratory rate (RR) and tidal volume (TV), however, this response is blunted by anaesthesia so it must be monitored and intervention may need to be initiated. Intervention is provided with either manual or mechanical positive pressure ventilation, which is discussed below.
An increase in MV (RR x TV) is required to aid in the removal of this excessive level of CO2. To reduce the risk of barotrauma by trying to increase the patient’s TV, increasing the RR should be the first action, and then the airway pressure and/or TV can then be increased if no improvement is seen.

A Flow Loop where loop-A shows positive pressure ventilation in the thorax at 10cmH2O to meet a tidal volume of 170ml, and then loop-B showing a pressure of 18cmH2O to reach the same tidal volume.
There is not only an extra-metabolic source of CO2 from insufflation but there may also be an increase in ETCO2 due to hypoventilation from the cranial displacement of the diaphragm, additionally contributing to a reduction in lung volume, functional residual capacity and airway compliance. As you can see, not only is there an increased absorption of CO2, but there is also a reduced elimination of CO2.
Most anaesthetists will mechanically ventilate laparoscopic patients however this may not be available in all veterinary practices. Young healthy cats may be able to ventilate effectively if the IAP is kept to 12mmHg (16cmH2O) (Beazley et al., 2011), and in healthy non-obese dogs, if the laparoscopic procedure is <30 minutes in duration with an insufflation pressure of 10mmHg, they may not require mechanical ventilation if the ETCO2 remains <55mmHg (Merlin et al., 2022).
Whilst some patients may not require positive pressure ventilation, it is advantageous to have the equipment to do so e.g. a ventilator, or in-circuit manometer, as some patients will not be able to compensate for the hypercapnia.
Pressure-cycled ventilation may be preferred to avoid an increased risk of barotrauma and high inflation pressures that may occur with pre-set tidal volumes when using volume-cycled ventilation modalities (Hayden and Cowman, 2011). The application of positive end expiratory pressures (PEEP) can be used to minimise alveolar de-recruitment however the blood pressure should be monitored in case this affects cardiac output further (Hayden and Cowman, 2011).
After abdominal deflation, dogs undergoing laparoscopic ovariectomies are usually able to increase their MV to produce more adequate ventilation, mostly through an increase in TV that returns to pre-insufflation values or exceeded them (Merlin et al., 2022).
Monitoring the ETCO2 non-invasively with capnography is useful but it is also important to consider there may be a difference between the ETCO2 and the arterial partial pressure of CO2 (PaCO2) resulting from a ventilation/perfusion (Q/V) mismatch. A V/Q mismatch occurs as pressure on the alveoli collapses them, reducing the surface area for gaseous exchange. Although there is no gas flow through them, they will still be perfused. If you suspect the patient has ‘trapped’ CO2 that is not being exhaled, an arterial blood sample can be obtained to monitor the PaCO2.
The use of pulse oximetry is recommended to ensure the patient is adequately oxygenating (SpO2 >95% or PaO2 >60mmHg). Hypoxemia is not a likely complication in patients breathing the high concentration of oxygen that is delivered during routine anaesthesia in small animal practice (de Rezende and Mama, 2015).
During the recovery period, patients may benefit from supplemental oxygen administration to mitigate the effects of pneumoperitoneum (Hayden and Cowman, 2011), especially as post-operative hypoxaemia can be seen in our veterinary patients already.
Analgesia
Patients should have analgesia appropriately administered for the procedure they are undergoing, with pain scoring and interventions being monitored postoperatively.
Local anaesthesia techniques can be provided for most surgical procedures (e.g. epidural, transversus abdominis plane blocks), as can a local infiltration of bupivacaine proximal to the skin incisions that the laparoscopic ports are inserted through (McNerney and Farry, 2015).
In human medicine, pain after laparoscopy has been described as intense but short-lived with the pain maximal during the first 2 hours post-anaesthesia (Hayden and Cowman, 2011), and we must assume the same in our veterinary species.
Air Embolism
A rare but serious complication is a major gas embolism due to the insufflator being incorrectly positioned, or the absorption of CO2 by a ruptured blood vessel (de Rezende and Mama, 2015). If microemboli pass through the right side of the heart, they will be eliminated via the lungs via exhalation. However larger emboli can lodge in the right atrium or ventricle, creating a gas lock which stops blood from moving through the heart.
In human medicine, a central venous catheter can be placed to remove the gas emboli, as does moving the patient into a left lateral position to “unlock” the emboli. These however have not been proven useful in veterinary medicine and are rapidly fatal.
Renal and Hepatic Changes
Blood flow can be reduced due to an increase in IAP and this should be considered in patients undergoing laparoscopic procedures that already have dysfunction of these organ systems, however, the most dramatic influences occur once IAPs >20mmHg (27 cmH2O) are used (Hayden and Cowman, 2011; de Rezende and Mama, 2015).
In dogs, hepatic transaminases increase for up to 48 hours post laparoscopic surgery with ALT and AST are proportional to the intraabdominal pressure and duration of insufflation (Nesek-Adam et al., 2004).
Neurological
There are increases in intracranial pressures due to the changes in cerebral venous drainage and raised intrathoracic pressures. There are reports in human medicine of neurological dysfunction in patients that have prolonged laparoscopic procedures, especially in those in a Trendelenburg position (Hayden and Cowman, 2011).
Airway Management
In veterinary anaesthesia, a cuffed endotracheal tube is commonly placed to secure the airway which allows not only effective ways to deliver positive pressure ventilation, but also protects the airway against gastric fluid aspiration. Therefore, there are controversial opinions about the use of a laryngeal mask airway (LMA) during laparoscopic surgery (Hayden and Cowman, 2011), which potentially could be extrapolated to the use of a v-gel® in veterinary patients undergoing the same procedure.
In human medicine, laparoscopic surgery has high incidences of postoperative vomiting and nausea, which may be prevented with prophylactic antiemetics (Hayden and Cowman, 2011).
Conclusions
It can be appreciated that insufflation of the abdomen during laparoscopy affects multiple physiological processes that the veterinary team should be aware of.
Routine monitoring equipment, such as electrocardiography, pulse oximetry, capnography and blood pressure monitoring should be used alongside intravenous fluid administration to monitor the effects of insufflation with CO2.
Recommended Reading
Fundamental Techniques in Laparoscopy – Chapter 7; Anesthesia Management of Dogs and Cats for Laparoscopy by Marlis L. de Rezende and Khursheed Mama.
References
Beazley, S., Cosford, K. and Duke-Novakovski, T., 2011. Cardiopulmonary effects of using carbon dioxide for laparoscopic surgery in cats. Canadian Veterinary Journal, 52(9), pp.973-978.
Buote, N., Kovak-McClaran, J. and Schold, J., 2010. Conversion from Diagnostic Laparoscopy to Laparotomy: Risk Factors and Occurrence. Veterinary Surgery, 40(1), pp.106-114.
Charlesworth, T. and Sanchez, F., 2019. A comparison of the rates of postoperative complications between dogs undergoing laparoscopic and open ovariectomy. Journal of Small Animal Practice, 60(4), pp.218-222.
Culp, W., Mayhew, P. and Brown, D., 2009. The Effect of Laparoscopic Versus Open Ovariectomy on Postsurgical Activity in Small Dogs. Veterinary Surgery, 38(7), pp.811-817.
de Rezende, M. and Mama, K., 2015. Anesthesia Management of Dogs and Cats for Laparoscopy. In: B. Fransson and P. Mayhew, ed., Small Animal Laparoscopy and Thoracoscopy, 1st ed. Hoboken, New Jersey: Wiley Blackwell.
Devitt, C., Cox, R. and Hailey, J., 2005. Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs. Journal of the American Veterinary Medical Association, 227(6), pp.921-927.
Doerfelt, R. and Eberspacher-Schweda, E., 2010. Anaesthesia for laparoscopic surgery in small animal practice. Der Praktische Tierarzt, 91(8), pp.640-647.
Fukushima, F., Malm, C., Andrade, M., Oliveria, H., Melo, E., Caldeira, F., Gheller, V., Palhares, M., Macedo, S., Figueiredo, M. and Silva, M., 2011. Cardiorespiratory and blood gas alterations during laparoscopic surgery for intra-uterine artificial insemination in dogs. The Canadian Veterinary Journal, 52(1), pp.77-79.
Hayden, P. and Cowman, S., 2011. Anaesthesia for laparoscopic surgery. Continuing Education in Anaesthesia Critical Care & Pain, 11(5), pp.177-180.
Hancock, R., Lanz, O., Waldron, D., Duncan, R., Broadstone, R. and Hendrix, P., 2005. Comparison of Postoperative Pain After Ovariohysterectomy by Harmonic Scalpel-Assisted Laparoscopy Compared with Median Celiotomy and Ligation in Dogs. Veterinary Surgery, 34(3), pp.273-282.
Henny, C. and Hofland, J., 2005. Laparoscopic surgery: Pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surgical Endoscopy, 19(9), pp.1163-1171.
McNerney, T. and Farry, T., 2015. Surgical Pain Management. In: M. Goldberg and N. Shaffron, ed., Pain Management for Veterinary Technicians and Nurses, 1st ed. Hoboken, New Jersey: John Wiley & Sons, Inc, p.106.
Merlin, T., Cinti, F. and Charlesworth, T., 2022. Healthy nonobese bitches maintain acceptable spontaneous ventilation during laparoscopic ovariectomies. Journal of the American Veterinary Medical Association, pp.1-7.
Nesek-Adam, V., Rasic, Z., Kos, J. and Vnuk, D., 2004. Aminotransferases after experimental pneumoperitoneum in dogs. Acta Anaesthesiologica Scandinavica, 48(7), pp.862-866.
Perilli, V., Sollazzi, L., Bozza, P., Modesti, C., Chierichini, A., Tacchino, R. and Ranieri, R., 2000. The Effects of the Reverse Trendelenburg Position on Respiratory Mechanics and Blood Gases in Morbidly Obese Patients During Bariatric Surgery. Anesthesia & Analgesia, 91(6), pp.1520-1525.
Schauvliege, S. (2016). Patient Monitoring and Monitoring Equipment. In: T. Duke-Novakovski, M. de Vries and C. Seymour, ed., BSAVA Manual of Canine and Feline Anaesthesia and Analgesia., 3rd ed. Gloucester: John Wiley & Sons, p.82